��Ŀ����
1����ǰ������Ⱦ����Խ��Խ���أ������ꡢ����ЧӦ���������ƻ����ж����ʵ���Ⱦ������β������Ⱦ��ˮ����Ⱦ���ѳ�Ϊ��������ձ��ע���о������⣮��ش�
��1���������������Ҫ�����Ǣ� ������ţ���ͬ����
��SO2 ��CO2
��2��ʹ�������ܵ��ƻ�����Ҫ�����Ǣڣ�
�ٶ�����̼ �ڷ��ȴ���
��3����ɾ�����Ⱦ�������Ǣ٣�
��CO ��N2
��4�����ˮ����Ⱦ��ԭ���Ǣ٢ڣ�
��������ˮ�������ŷ� �ڹ�ҵ��ˮ�������ŷ�
��5��Ϊ������������Ⱦ���⣬��������������������װһ������ת���������ò����ٺϽ��������������ص���ʹ�����ŷų���CO��NO��Ӧ������CO2��N2��д���÷�Ӧ�Ļ�ѧ����ʽ��2CO+2NO $\frac{\underline{\;����\;}}{\;}$ 2CO2+N2��
��6��ú����������Ч�ؽ��ʹ�������Ⱦ����ŷţ��Ǹ�Ч����������ú̿����Ҫ;����һЩ������ʹ�õ�ˮú���ǽ���̿��ˮ�����ڸ����·�Ӧ�õ��ģ��÷�Ӧ�Ļ�ѧ����ʽ��C+H2O��g��$\frac{\underline{\;����\;}}{\;}$ CO+H2����
���� ��1��������������������ŷ��������������Ҫԭ��
��2���ƻ�������������Ƿ�������
��3��CO�ж�������Ϊ������Ҫ�ɷ֣�
��4�����ˮ����Ⱦ��ԭ���ǣ���ҵ��Ⱦ��ũҵ��Ⱦ��������Ⱦ��
��5��һ����̼��һ�������ڴ������������ɵ�����ˮ��
��6��̼��ˮ������Ӧ����������һ����̼��
��� �⣺��1���������������Ҫ�����Ƕ�������
��ѡ���٣�
��2���ƻ�������������Ƿ�������
��ѡ���ڣ�
��3��CO�ж�������Ϊ������Ҫ�ɷ֣�
��ѡ���٣�
��4�����ˮ����Ⱦ��ԭ���ǣ���ҵ��Ⱦ��ũҵ��Ⱦ��������Ⱦ��
��ѡ���٢ڣ�
��5��һ����̼��һ�������ڴ������������ɵ�����ˮ������ʽ��2CO+2NO $\frac{\underline{\;����\;}}{\;}$ 2CO2+N2��
�ʴ�Ϊ��2CO+2NO $\frac{\underline{\;����\;}}{\;}$ 2CO2+N2��
��6��̼��ˮ������Ӧ����������һ����̼������ʽ��C+H2O��g��$\frac{\underline{\;����\;}}{\;}$ CO+H2��
�ʴ�Ϊ��C+H2O��g��$\frac{\underline{\;����\;}}{\;}$ CO+H2��
���� ���⿼���������г�������Ⱦ����������Ϥ�й����ʵ������ǽ���ؼ�������������ѧ���������û�����ʶ��

 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�| A�� | ���Ȼ��ƾ�����ÿ��Na+����Cl-����Χ������8��Cl-��Na+�� | |
| B�� | ԭ�Ӿ�����ֻ���ڹ��ۼ� | |
| C�� | ���Ӿ���ͷ��Ӿ������ۻ�ʱ����ѧ�������ƻ� | |
| D�� | ����״̬���ܵ���ľ���һ���ǽ������� |
| A�� | C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.5 kJ/mol | |
| B�� | H2��g��+Cl2��g��=2HCl��g����H=-184.6 kJ/mol | |
| C�� | H2��g��+$\frac{1}{2}$O2��g��=H2O��g����H=-241.8 kJ/mol | |
| D�� | C8H18��l��+$\frac{25}{2}$O2��g��=8CO2��g��+9H2O��l����H=-5518 kJ/mol |
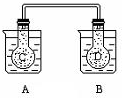 ��ͼ��ʾ���ڳ���������A��ʢ��500mL����ˮ��������B��ʢ�� 500ml 1mol/L�����ᣬ����ƿC��D�г��������������壬���õ��ܽ�������ͨ����A�����м���50g����茶��壬С�Ľ��裬ʹ��Ѹ���ܽ⣻��B�м���10g�����ƹ��壬С�Ľ���Ҳʹ��Ѹ���ܽ⣬����A��B�й������ʵ��ܽ⣬��ƿC��D���������ɫ�仯�ǣ�������
��ͼ��ʾ���ڳ���������A��ʢ��500mL����ˮ��������B��ʢ�� 500ml 1mol/L�����ᣬ����ƿC��D�г��������������壬���õ��ܽ�������ͨ����A�����м���50g����茶��壬С�Ľ��裬ʹ��Ѹ���ܽ⣻��B�м���10g�����ƹ��壬С�Ľ���Ҳʹ��Ѹ���ܽ⣬����A��B�й������ʵ��ܽ⣬��ƿC��D���������ɫ�仯�ǣ�������| A�� | ���� | B�� | ��ƿC����ɫ���D�б�dz | ||
| C�� | ��ƿD�б��C�б�dz | D�� | ������ƿ�е���ɫ������ |
��ʵ��Ŀ�ģ�̽��ͬһ����Ԫ�����ʵĵݱ���ɣ�
��ʵ����Ʒ���⻯����Һ���廯����Һ����ˮ����ˮ�����Ȼ�̼��
��������1���Թܣ���2����ͷ�ιܣ�������д������Ҫ�IJ���������
��ʵ�����ݣ�
| ��� | ʵ�鷽�� | ʵ������ |
| �� | ��������ˮ����ʢ������KBr��Һ���Թ��У����ٵ����������Ȼ�̼���� | ������ˮ����Һ����ɫ��Ϊ��ɫ���������Ȼ�̼��ˮ����ɫ��dz�����Ȼ�̼�㣨�²㣩��Ϊ�Ⱥ�ɫ |
| �� | ��������ˮ����ʢ������NaI��Һ���Թ��У����ٵ����������Ȼ�̼���� | ������ˮ����Һ����ɫ��Ϊ��ɫ���������Ȼ�̼��ˮ����ɫ��dz�����Ȼ�̼�㣨�²㣩��Ϊ��ɫ |
������������ۣ�
�����ýṹ���ۼ�˵���ó��������۵�ԭ��4��ԭ�Ӱ뾶Խ��õ�������Խ��
������F2��ͬNa���ڻ��ã��������һ�����û���Ӧʵ����֤��������ǿ�������о�һ����ʵ˵��F�ķǽ����Ա�Clǿ����5���ֱ���������Ӧ��F2�����ף�

