��Ŀ����
3��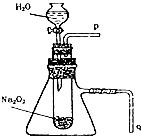 ����֬����סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ��������
����֬����סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������1����ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ�����ac������ţ�
a����Ӧ���� b�����ڿ����п�����ȼ
c����O2���� d��Na2O2�ڿ����п�����ȼ
��2��д��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ2Na2O2+2H2O=4NaOH+O2����
��3��ijУ�о���ѧϰС��������ͼװ�ý���ʵ�飬��֤���������ۣ�Ϊ��֤�õ����������������ʵ����������������ǣ����������ţ����Թ��ڰ��й������Ƶ�ʯ�����ϵμ���ˮ���ô����ǵ�ľ������p�������ľ����ȼ�������������ɣ���֮���������ɣ�
���� ��1���۲쵽��֬����ȼ����������֪����������ˮ��Ӧ�����������ҷų�����������������ȼ��
��2��Na2O2��H2O��Ӧ����NaOH��������
��3������������ȼ�ԣ��Դ������
��� �⣺�� 1����֬����ȼ��˵���߱�ȼ�յ��������Թ��ڿ������٣�������ȼ��Ӧ���д���������ֻ�и÷�Ӧ�Ƿ��ȷ�Ӧ����ʹȼ���¶ȴﵽ�Ż�㣬��������֪�÷�Ӧ�����������ҷ��ȣ��ʴ�Ϊ��ac��
��2���������ƺ�ˮ��Ӧ�����������ƺ�����������ʽΪ2Na2O2+2H2O=4NaOH+O2�����ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
��3����������ʹ�����ǵ�ľ����ȼ�����ʣ����Կ��ô����ǵ�ľ������p�������ľ����ȼ�������������ɣ���֮���������ɣ�
�ʴ�Ϊ�����������ţ����Թ��ڰ��й������Ƶ�ʯ�����ϵμ���ˮ���ô����ǵ�ľ������p�������ľ����ȼ�������������ɣ���֮���������ɣ�
���� ���⿼��������Ƶ����ʼ�ʵ��װ�õ�Ӧ�ã�Ϊ��Ƶ���㣬���չ���������ˮ�ķ�Ӧ������������Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע����������۵Ĺ�ϵ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
�����Ŀ
5�����й��ڻ�ѧ���������������ʶ��ȷ���ǣ�������
| A�� | ʵ�������ۻ���������ʱ����ѡ��ʯӢ������������ | |
| B�� | �����̼���ȡ�ġ��ع��͡����й̶����۷е� | |
| C�� | ʳƷ����Ĥ�����ʿɷ�Ϊ����ϩ��PE����������ϩ��PVC������ƫ������ϩ��PVDC���ȣ�PVC�ĵ������PE�ĵ������Ȼ���ӳ��Ƶ� | |
| D�� | ��ͥ��ʳ�ô״���CO2����ǿƯ�۵�Ư���� |
8��ʵ������������װ�ý�����Ӧʵ�飬�ܴﵽʵ��Ŀ���ǣ�������
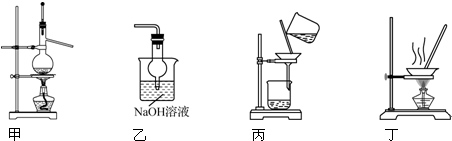

| A�� | ��װ�ü����Ҵ���Ũ����Ϊԭ������ϩ | |
| B�� | ��װ��������ijЩʵ��β���еĶ������� | |
| C�� | ��װ�ñ�����Cl2��KI��Һ��Ӧ���ɵĵ� | |
| D�� | ��װ�ö�����NH4Cl������Һ��ȡNH4Cl���� |
12�������£���������������Һ�������У���ȷ���ǣ�������
| ��� | �� | �� | �� | �� |
| ��Һ | ��ˮ | NaOH | CH3COOH | HCl |
| Ũ��c/mol•L-1 | 0.01 | 0.01 | 0.01 | 0.01 |
| A�� | ��ˮ�������c��H+������=��=��=�� | |
| B�� | ��Һ��pH���ڣ��٣��ܣ��� | |
| C�� | �ڡ��ۻ�ϳ����ԣ�������Һ��������ۣ��� | |
| D�� | �١��ܵ������ϣ�������Һ�����ӵ�Ũ�ȣ�c��NH4+��=c��Cl-����c��H+��=c��OH-�� |
13����ѧ����ᡢ����������أ�����е���ʵ�ͽ��;���ȷ���ǣ�������
| ѡ�� | ��ʵ | ���� |
| A | ���ȵĴ�����Һϴȥ���� | Na2CO3����Ϊ��֬�ֽ�Ĵ��� |
| B | ��������������ϡ���� | �������γ���������Ĥ���б������� |
| C | ������Ϊ��ױƷ�еı�ʪ�� | ��������ˮ�γ���� |
| D | ������ʴˮ������Ʒ | HF����ǿ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
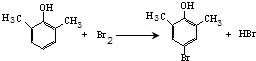 ��
�� ��
�� ��
�� ��
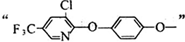
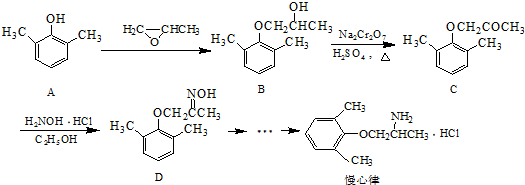
��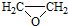 ����д�����ڼ����� ��
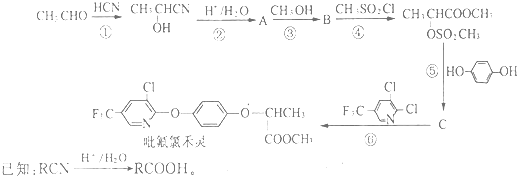
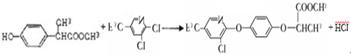
����д�����ڼ����� ��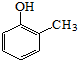 �����Ҵ�Ϊԭ���Ʊ�
�����Ҵ�Ϊԭ���Ʊ�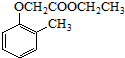 �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���
 ��
��