��Ŀ����
18������˵����ȷ���ǣ����������ڱ�״���£������Ħ�����Ϊ22.4L
���ڱ�״���£�1mol�κ�����������Լ��22.4L
��1molˮ�ڱ���µ����ԼΪ22.4L
��1.5molN2��0.5molO2�ڱ�״���»�����ԼΪ44.8L
��1molH2��������2g������ռ�������22.4L
����ͬ��ͬѹʱ����ͬ������κ����嵥��������ԭ����Ŀ��ͬ��
| A�� | �٢ڢۢ� | B�� | �ڢ� | C�� | �ܢ� | D�� | �ܢ� |
���� �������Ħ������ĵ�λΪL/mol��
���ڱ�״���£������Ħ�����Ϊ22.4L/mol��
���ڱ�״����ˮΪҺ�壻
��1.5molN2��0.5molO2��ϲ���Ӧ�������ʵ���Ϊ2mol��
��û��˵���DZ�״���£������������
����ͬ��ͬѹʱ����ͬ������κ����嵥�������ķ�����Ŀ��ͬ��
��� �⣺�������Ħ������ĵ�λΪL/mol�����ڱ�״���£������Ħ�����Ϊ22.4L/mol���ʴ���
���ڱ�״���£������Ħ�����Ϊ22.4L/mol�������ڱ�״���£�1mol�κ�����������Լ��22.4L������ȷ��
���ڱ�״����ˮΪҺ�壬����1molˮ�ڱ���µ��������22.4L���ʴ���
��1.5molN2��0.5molO2��ϲ���Ӧ�������ʵ���Ϊ2mol���ڱ�״���£������Ħ�����Ϊ22.4L/mol����2mol��������Ϊ44.8L������ȷ��
��1molH2��������2g��û��˵���DZ�״���£�������������ʴ���
����ͬ��ͬѹʱ����ͬ������κ����嵥�������ķ�����Ŀ��ͬ����ԭ������һ����ͬ������ͬ�����O2��O3������ԭ������ͬ���ʴ���
��ѡB��
���� ���⿼��������Ħ�������Ӧ�ã���Ŀ�ѶȲ���ע���������Ħ������ĵ�λ��������������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ̼���ƣ�CaC2���ĵ���ʽ��Ca2+[��C����C��]2- | |
| B�� | ${\;}_{8}^{18}$O2-���ӵĽṹʾ��ͼ��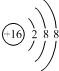 | |
| C�� | 2-��-2-�����Ľṹ��ʽ��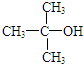 | |
| D�� | Na2Sˮ������ӷ���ʽ��S2-+2H2O?2H2S+2OH- |
| A�� | ������CH4��CO2�����庬����������������ЧӦ�Ӿ磬������������������ | |
| B�� | �����������ոѻ�����������������͡�����Ϊ��ͬ���ķ�ɢϵ | |
| C�� | ̫����ֽ�ˮ��������ֲ��ո����������������Ҵ����漰���������ܵ����� | |
| D�� | PbO2���������Ժ͵����ԣ�������Ǧ������������ |
| A�� | ��ˮϡ�ͣ�����ƽ�������ƶ�����Һ��n��H+����С | |
| B�� | ���ȣ�����ƽ�������ƶ�����Һ��c��CH3COO-������ | |
| C�� | ͨ������HCl���壬����ƽ�������ƶ�����Һ��c��H+����С | |
| D�� | ��������CH3COONa���壬����ƽ�������ƶ�����Һ��c��H+����С |
| A�� | �ƵĻ�ѧ���ʺܻ��ã�����Ȼ���ﲻ��������̬���� | |
| B�� | ��ѧ������������ʶ���� | |
| C�� | �ڻ�ѧ��Ӧ�У��μӷ�Ӧ�ĸ����ʵ������ȵ��������ʵ���֮�� | |
| D�� | ������ѧ���Ž��з����ԭ��ѧ˵��Ϊ������ѧ�ķ�չ�춨�˼�ʵ�Ļ��� |
| A�� | ����ʽΪC6H12�����ӽṹ�к���3����-CH3��ԭ���ŵ�ϩ������ 5�� | |
| B�� | ���Ӵ���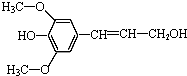 �� �ܷ���������ȡ����ˮ�⡢�Ӿ۷�Ӧ �� �ܷ���������ȡ����ˮ�⡢�Ӿ۷�Ӧ | |
| C�� | ʵ��������ϩʱ�������ɵ�����ͨ������KMnO4��Һ����ɫ��ȥ������˵��һ����������ϩ | |
| D�� | ������ij���ʵ���Һ�μӵ�����������Һ�У�ˮԡ���Ⱥ����������ɣ������ʲ�һ������ȩ�� |
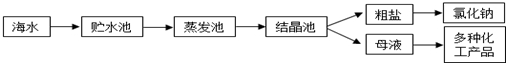
��1���ٴ����к���Ca2+��Mg2+��SO42-�����ʣ�����ʱ�����Լ�Ϊ��A�����B��BaCl2��Һ��C��NaOH��Һ��D��Na2CO3��Һ�������Լ���˳����CBDA��BCDA��
�ڵ�ⱥ��ʳ��ˮʱ�����Դ���������ĵ缫�Ϸ����ķ�ӦΪ2Cl--2e-=Cl2�������Դ���������ĵ缫������ҺpH�ı仯�DZ��
��2����ҵ�ϴӺ�ˮ����ȡ��NaCl����������ȡ������Ҫ�������£���ʳ��ˮ����ͨ������A����ͨ������B����ַ�Ӧ����˵õ�����C����ҺD��������C���ռ����Ƶô��������B��CO2���ѧʽ������NaCl��ȡ���Ӧ�Ļ�ѧ����ΪNaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+CO2��+H2O��
��3����ˮ��þ��һ�ι���������ͼ��
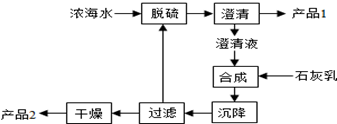
Ũ��ˮ����Ҫ�ɷ����£�
| ���� | Na+ | Mg2+ | Cl- | SO42- |
| Ũ��/��g•L-1�� | 63.7 | 28.8 | 144.6 | 46.4 |
| A�� | 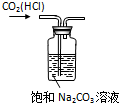 ��CO2�е�HCl | B�� | 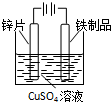 ����Ʒ�϶�п | C�� |  ���հ��� | D�� |  �Ʊ�����O2 |
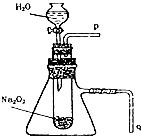 ����֬����סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ��������
����֬����סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ��������