��Ŀ����
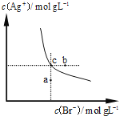
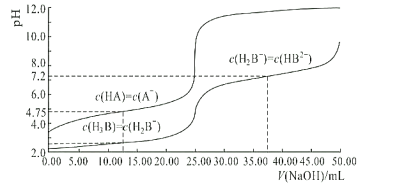
����Ŀ��25��ʱ�������Ϊ25.00mL��Ũ�Ⱦ�Ϊ0.0100mol/L��HA��H3B��Һ�ֱ���0.0100mol/LNaOH��Һ�ζ�����Һ��pH��V(NaOH)�仯������ͼ��ʾ������˵������ȷ���ǣ� ��
A.NaOH��Һ�ζ�HA��Һ��ѡ������ָʾ��
B.��Ϊ0.0100mol/LHA��H3B��Һ�У����Խ�ǿ����HA
C.25��ʱ��0.0100mol/LNa2HB��Һ��pH��7
D.25��ʱ��H2B-���ӵ�ˮ�ⳣ����������Ϊ10-3
���𰸡�C
��������
A���ζ��յ�����ǿ�������Σ���Һ�ʼ��ԣ������ȵı�ɫ��Χ��3.1~4.4������Ӧѡ��̪��ָʾ������A����
B����ͼ��֪��Ũ�Ⱦ�Ϊ0.0100mol/LHA��H3B��Һ�У�HA��H3B��Һ��H3B��Һ��ʼʱpH��С��˵��H3B����������ӵ�����ǿ��HA�������Խ�ǿ��ΪH3B����B����
C��![]() ��
��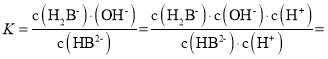
![]() ����ͼ��֪
����ͼ��֪![]() ʱ��pHΪ7.2����Ka2=10-7.2��K=10-6.8��HB2-�ĵ��볣��ΪKa3������Ka2>>Ka3�����Գ�����HB2-��ˮ��̶ȴ��ڵ���̶ȣ�NaH2B��Һ�ʼ��ԣ���C��ȷ��
ʱ��pHΪ7.2����Ka2=10-7.2��K=10-6.8��HB2-�ĵ��볣��ΪKa3������Ka2>>Ka3�����Գ�����HB2-��ˮ��̶ȴ��ڵ���̶ȣ�NaH2B��Һ�ʼ��ԣ���C��ȷ��
D��![]() ��K=
��K=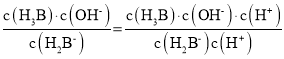
![]()
=![]() ����ͼ��֪��
����ͼ��֪��![]() ʱ��������Ũ�ȵ���Ka1�����Ka1��������10-2~10-3��H2B-���ӵ�ˮ�ⳣ����������Ϊ10-12����D����
ʱ��������Ũ�ȵ���Ka1�����Ka1��������10-2~10-3��H2B-���ӵ�ˮ�ⳣ����������Ϊ10-12����D����
��ѡC��

��ϰ��ϵ�д�
�����Ŀ