��Ŀ����
����Ŀ����N��P��Ti��Ԫ����ɵ����Ͳ������Ź㷺����;����ش��������⡣
��1����Ԫ�ػ�̬ԭ��δ�ɶԵ�����Ϊ__����������ߵĵ���ռ�ݵ��ܼ�����Ϊ___��
��2����һ��ͬ��������������(P4)�����幹��Ϊ__���Ʋ�����CS2�е��ܽ��__(��������������С����)��ˮ�е��ܽ�ȡ�
��3��������������̬�⻯���(PH3)�Ͱ�(NH3)�ļ��Ƿֱ�Ϊ93.6����107�����Է���PH3�ļ���С��NH3��ԭ��__��
��4����ҵ���ƽ����Ѳ��ý�����ԭ���Ȼ��ѡ��Ƚ�TiO2(����Ȼ�Ľ��ʯ)������̿�ۻ�ϼ�����1000��1100K�������Ȼ�����������TiCl4��д������TiCl4�Ļ�ѧ��Ӧ����ʽ��___��
��5����һ�ֵ����Ѿ���ľ�����ͼ��ʾ���þ���Ļ�ѧʽΪ__����֪������ܶ�Ϊ��g��cm-3�������ӵ�����ΪNA�����߳�Ϊ__cm(�ú�����NA��ʽ�ӱ�ʾ)��
���𰸡�2 3d ���������� ���� �縺��Nǿ��P������ԭ�ӵĵ縺��Խ�ɼ����Ӷ�������ԭ��Խ�����ɼ����Ӷ�֮�����ԽС���ɼ����Ӷ�֮����ų��������DZ�� TiO2��2C��2Cl2![]() TiCl4��2CO TiN
TiCl4��2CO TiN ![]()
��������
(1)������ԭ�ӵĺ�������Ų�ʽ���
(2)���ݰ��Ľṹ��𣻸�����������ԭ�����
(3)���ݵ縺�ԶԳɼ����ӶԵ�Ӱ����
(4)���ݷ�Ӧ�����������ԭ���غ���д����ʽ��
(5)���ݾ�̯�����㡣
(1)��Ԫ�ػ�̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d24s2���������δ�ɶԵ�����Ϊ2����������ߵĵ���ռ�ݵ��ܼ�����Ϊ3d���ʴ�Ϊ��2��3d��
(2)����(P4)�����к���6��P��P�����������幹��Ϊ���������Σ�����CS2��Ϊ�Ǽ��Է��ӣ�ˮ�Ǽ��Է��ӣ�������������ԭ�����Ʋ������CS2�е��ܽ�ȴ�����ˮ�е��ܽ�ȣ��ʴ�Ϊ�����������Σ����ڣ�
(3)���ڵ縺��Nǿ��P������ԭ�ӵĵ縺��Խ�ɼ����Ӷ�������ԭ��Խ�����ɼ����Ӷ�֮�����ԽС���ɼ����Ӷ�֮����ų��������DZ�����PH3�ļ���С��NH3�ģ��ʴ�Ϊ���縺��Nǿ��P������ԭ�ӵĵ縺��Խ�ɼ����Ӷ�������ԭ��Խ�����ɼ����Ӷ�֮�����ԽС���ɼ����Ӷ�֮����ų��������DZ��
(4)��Ӧ����������TiO2������̿�ۣ���������TiCl4������ԭ���غ���̼������֪����CO���ɣ�������TiCl4�Ļ�ѧ��Ӧ����ʽΪTiO2��2C��2Cl2![]() TiCl4��2CO���ʴ�Ϊ��TiO2��2C��2Cl2
TiCl4��2CO���ʴ�Ϊ��TiO2��2C��2Cl2![]() TiCl4��2CO��
TiCl4��2CO��
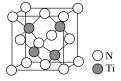
(5)���ݾ����ṹ��֪���е�Nԭ�Ӹ�����8��1/8��6��1/2��4��Tiԭ��ȫ���ھ����У�������4������þ���Ļ�ѧʽΪTiN���辧���ı߳���acm����![]() ���������a��
���������a��![]() ���ʴ�Ϊ��TiN��
���ʴ�Ϊ��TiN��![]() ��
��

����Ŀ����H2O2��KI��ϴ�ྫ����ɡ��������ࡱʵ�飨��ʱ���ڲ���������ĭ����ijͬѧ�����������϶Ը�ʵ�����̽����
��1������1��KI�ڸ÷�Ӧ�е����ã�
H2O2��I����H2O��IO����H2O2��IO����H2O��O2����I�����ܷ�Ӧ�Ļ�ѧ����ʽ��________________��
��2������2��H2O2�ֽⷴӦ�����������仯��ͼ��ʾ�����Т���KI���룬����KI���롣�����ж���ȷ����___________������ĸ����
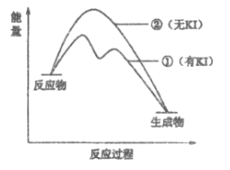
a. ����KI��ı��˷�Ӧ��·��
b. ����KI��ı����ܷ�Ӧ�������仯
c. H2O2��I����H2O��IO���Ƿ��ȷ�Ӧ
��3��ʵ���з��֣�H2O2��KI��Һ��Ϻ����������ݣ���Һ��ɫ��ơ��ټ���CCl4�������ã��������Լ��١�
����3��I2Ҳ�ɴ�H2O2�ķֽⷴӦ��
�ټ�CCl4�������úɹ۲쵽___________��˵����I2���ɡ�
���������Լ��ٵ�ԭ������ǣ�i. H2O2Ũ�Ƚ��ͣ�ii. ________�����¶���ʵ��˵��i������Ҫԭ����H2O2��Һ�м���KI��Һ������Һ��ƺֳ����ȷ���A��B���Թ��С�A�Թܼ���CCl4��B�Թܲ���CCl4���ֱ������á��۲쵽��������_____________��
��4������4��I����aq����I2��aq��![]() I3����aq�� K��640��
I3����aq�� K��640��
Ϊ��̽����ϵ�к������Ĵ�����ʽ������ʵ�飺��20 mLһ��Ũ�ȵ�H2O2��Һ�м���10mL 0.10mol��L��1 KI��Һ����ƽ��������Ũ�����£�
�� | I�� | I2 | I3�� |
Ũ��/��mol��L��1�� | 2.5��10��3 | a | 4.0��10��3 |
��a��__________��
�ڸ�ƽ����ϵ�г��˺���I����I2��I3���⣬һ��������������������������________________��