��Ŀ����
����Ŀ��ʵ��������NaOH��������1.0 mol��L��1��NaOH��Һ240 mL��
(1)������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ������ڼ��㡡���ܽ⡡��ҡ�ȡ���ת�ơ���ϴ�ӡ��߶��ݡ�����ȴ����ҡ��
����ȷ�IJ���˳��Ϊ__________________�������õ��IJ����������ձ�����ͷ�ιܡ�________________��
(2)ijͬѧ������NaOH��������������������ƽ�����ձ�����������ƽƽ����״̬��ͼ��ʾ���ձ���ʵ������Ϊ________ g��Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ�________ g NaOH��

(3)ʹ������ƿǰ������е�һ��������________��
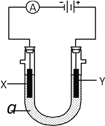
(4)��ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ��������������д����

��________________________________________________________________________
��________________________________________________________________________
(5)�����ƹ����У���������������ȷ�ģ����в������������ƫ�ߵ���________(����ĸ)��
A������NaOH�Ѿ�����
B��������ƿ�м�ˮδ���̶���
C��������NaOH��Һ�������ձ���
D���ô������������ƽ��5.4 g NaOH(1 g����������)ʱ����������������������
���𰸡��ڢ٢ۢ�ݢޢ�ߢ� 250 mL����ƿ ������ 27.4 10.0 ��© Ӧ��Ϊ250mL������ƿ����ҺʱӦ�ò����������� B
��������
��1������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ǩ��������ȷ��˳��Ϊ���ڢ٢ۢ�ݢޢߢܣ��õ�����������Ϊ��������ƽ��ҩ�ס����������ձ�������ƿ�ͽ�ͷ�ιܣ�Ҫ����1.0mol/L��NaOH��Һ240mL��Ӧѡ��250mL����ƿ�����Ի�ȱ�ٵ�������250mL����ƿ�����������ʴ�Ϊ���ڢ٢ۢ�ݢޢ�ߢ���250mL����ƿ����������
��2��������ƽ��������ԭ����֪����������=���������+���������������������Ϊ20g��10g����������2.6g�������ձ���ʵ������Ϊ27.4g������1.0molL-1��NaOH��Һ240mL��Ӧѡ��250mL����ƿ������250mL��Һ����Ҫ������������m=1.0mol/L��40g/mol��0.25L=10.0g���ʴ�Ϊ��27.4��10.0��
��3������ƿ���л�����ʹ�ù�������Ҫ���µߵ�������ʹ��ǰӦ����Ƿ�©ˮ���ʴ�Ϊ����©��
��4������ͬѧѡ�õ�����ƿ����Ӧ��Ϊ250mL������ƿ������ҺʱӦ�ò�������������Ϊ��Ӧ��Ϊ250mL������ƿ����ҺʱӦ�ò�����������
��5��A.NaOH�ѳ��⣬�������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���A������
B.����Һ�м�ˮδ���̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���B��ȷ��
C.�ܽ��NaOH�����������ձ��У��������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���C����
D.����NaOH���ŷ�ʱ��������ʵ������Ϊ4.6g���������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���D����ΪB��
��1������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ǩ��������ȷ��˳��Ϊ���ڢ٢ۢ�ݢޢߢܣ��õ�����������Ϊ��������ƽ��ҩ�ס����������ձ�������ƿ�ͽ�ͷ�ιܣ�Ҫ����1.0mol/L��NaOH��Һ240mL��Ӧѡ��250mL����ƿ�����Ի�ȱ�ٵ�������250mL����ƿ�����������ʴ�Ϊ���ڢ٢ۢ�ݢޢ�ߢ���250mL����ƿ����������
��2��������ƽ��������ԭ����֪����������=���������+���������������������Ϊ20g��10g����������2.6g�������ձ���ʵ������Ϊ27.4g������1.0molL-1��NaOH��Һ240mL��Ӧѡ��250mL����ƿ������250mL��Һ����Ҫ������������m=1.0mol/L��40g/mol��0.25L=10.0g���ʴ�Ϊ��27.4��10.0��
��3������ƿ���л�����ʹ�ù�������Ҫ���µߵ�������ʹ��ǰӦ����Ƿ�©ˮ���ʴ�Ϊ����©��
��4���ٸ�ͬѧѡ�õ�����ƿ����Ӧ��Ϊ250mL������ƿ������ҺʱӦ�ò�������������Ϊ��Ӧ��Ϊ250mL������ƿ����ҺʱӦ�ò�����������
��5��A.NaOH�ѳ��⣬�������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���A����
B.����Һ�м�ˮδ���̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���B��ȷ��
C.�ܽ��NaOH�����������ձ��У��������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���C����
D.����NaOH���ŷ�ʱ��������ʵ������Ϊ4.6g���������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���D����ΪB��

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�