��Ŀ����
11����NAΪ�����ӵ�������ֵ������������ȷ���ǣ�������| A�� | ��״���£�33.6 L �������к��з�ԭ�ӵ���ĿΪ1.5NA | |
| B�� | pH��Ϊ5��NH4Cl��NaHSO4��Һ��ˮ���������������Ŀ��Ϊ10-5NA | |
| C�� | ��1L 0.1 mol•L-1CuSO4��Һ�У���������������0.1 NA | |
| D�� | ij�ܱ�����ʢ��0.1molN2��0.3molIH2����һ�������³�ַ�Ӧ��ת�Ƶ��ӵ���ĿΪ NA |
���� A������£�HFΪҺ̬��
B����Һ�������ȷ��
C��SO42-�����ʵ���Ϊ0.1mol����ˮ���ܵ����OH-��
D��N2��H2�ķ�Ӧ�ǿ���ģ����ܽ��г��ף�
��� �⣺A������£�HFΪҺ̬������������Ħ����������㣬��A����
B����Һ�����ȷ��������Һ�е������ӵĸ��������㣬��B����
C��1L 0.1 mol•L-1CuSO4��Һ��SO42-�����ʵ���Ϊ0.1mol������Һ�л���ˮ�������OH-������Һ�е���������������0.1NA������C��ȷ��
D��N2��H2�ķ�Ӧ�ǿ���ģ����ܽ��г��ף���ת�Ƶĵ�����С��0.6NA������D����
��ѡC��
���� ���⿼���˰���٤���������йؼ��㣬�������չ�ʽ��ʹ�ú����ʵĽṹ�ǽ���ؼ����ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
1�����ϲ��ϵ��ŵ��ǣ�������
��ǿ�ȸ� �������� ������ ����ʴ��
��ǿ�ȸ� �������� ������ ����ʴ��
| A�� | ���٢� | B�� | ���ڢ� | C�� | ������ | D�� | �٢ڢۢ� |
2����������105B��115B����ͬλ��ԭ�ӹ��ɣ���֪5.4g������ȫ��ת����B2H6�����飩����ʱ���ɵñ�״����5.6L���飬������˵����ȷ���ǣ�������
| A�� | ������105B��115B����ͬλ��ԭ�ӵ�������Ϊ1��4 | |
| B�� | 5.4 g�þ�������������Ϊ2.9 mol | |
| C�� | �������ķֱ���105B��115B���ɵľ���������������֮��Ϊ6��5 | |
| D�� | ��̼ԭ������Ϊw g����105Bԭ�ӵ�����Ϊ10w g |
6���ڱ�״���£��������������ƿ�ڷֱ�װ�и��������NH3����һ��������Ȼ������壻NO2��O2�����Ϊ4��1�Ļ�����壮Ȼ��ֱ�����Ȫʵ�飬������ƿ��������Һ�����ʵ���Ũ��֮��Ϊ��������
| A�� | 2��1��2 | B�� | 5��5��4 | C�� | 1��1��1 | D�� | ��ȷ�� |
 3-������
3-������ 2��2��3-��������
2��2��3-��������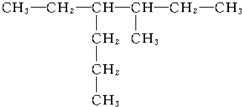 3-��-4-�һ�����
3-��-4-�һ����� 2��5-����-3-�һ�����
2��5-����-3-�һ����� 3��4-����-5-�һ�����
3��4-����-5-�һ����� 3-��-4-�һ����飮
3-��-4-�һ����飮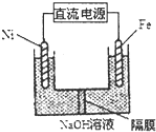
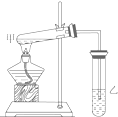 ��ͼ��ijѧ����Ƶ���ȡ����������ʵ��װ��ͼ��ʵ���в�ȡ��������Ҫʵ�������
��ͼ��ijѧ����Ƶ���ȡ����������ʵ��װ��ͼ��ʵ���в�ȡ��������Ҫʵ�������