��Ŀ����
[��ѧ��ѡ��3�����ʽṹ������]��15�֣�
��A��B��C��D��E��F��G��Hԭ������������ǰ�����ڰ���Ԫ�ء������������Ϣ���ش����⣺
��1��GԪ�ػ�̬ԭ�ӵļ۵����Ų�ͼΪ ��
��2�� ��Bԭ�Ӳ�ȡ���ӻ��������Ϊ ��
��Bԭ�Ӳ�ȡ���ӻ��������Ϊ ��
��3�����ݵȵ�����ԭ�����Ʋ� ���ӵĿռ乹��Ϊ ��
���ӵĿռ乹��Ϊ ��
��4�������й�E��F��������ȷ���ǣ� ��
a.���Ӱ뾶E��F b.�縺��E��F
c.���ʵ��۵�E��F d. E��F�ĵ��ʾ�������������û�
e. E��������������� f. E��F��������Ԫ�ع������Ӿ���
��5�� ��������
�������� ,ԭ���� ��
,ԭ���� ��
��6��E���ʵľ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ����������ͼ����ʾ����E���ʵľ���ѻ�ģ��Ϊ ��
����֪Eԭ�Ӱ뾶Ϊr pm�� ��ʾ����٤�������������ı߳�Ϊa�������ĸ�Ϊh�������ܶȿɱ�ʾΪ g/cm3������ֻ��r��
��ʾ����٤�������������ı߳�Ϊa�������ĸ�Ϊh�������ܶȿɱ�ʾΪ g/cm3������ֻ��r�� ����ʽ��ʾ��
����ʽ��ʾ��

��A��B��C��D��E��F��G��Hԭ������������ǰ�����ڰ���Ԫ�ء������������Ϣ���ش����⣺
| ��A��B��C��D��E��FΪ����������Ԫ�أ�ԭ�Ӱ뾶��С��ϵΪA��D��C��B��F��E�� |
| ��A��D�γɵĻ����ﳣ����ΪҺ̬�� |
| ��BԪ��ԭ�Ӽ۵��ӣ���Χ���ӣ��Ų�ΪnSnnPn |
| ��FԪ��ԭ�ӵĺ���p����������s����������1�� |
| �ݵ�һ�����ܣ�F��E�� |
| ��G�Ļ�̬ԭ�Ӻ�����6��δ�ɶԵ��ӣ� |
��H���γɺ�ɫ����ש��ɫ���� �ͺ�ɫ��HD���ֻ���� �ͺ�ɫ��HD���ֻ���� |
��2��
 ��Bԭ�Ӳ�ȡ���ӻ��������Ϊ ��
��Bԭ�Ӳ�ȡ���ӻ��������Ϊ ����3�����ݵȵ�����ԭ�����Ʋ�
 ���ӵĿռ乹��Ϊ ��
���ӵĿռ乹��Ϊ ����4�������й�E��F��������ȷ���ǣ� ��
a.���Ӱ뾶E��F b.�縺��E��F
c.���ʵ��۵�E��F d. E��F�ĵ��ʾ�������������û�
e. E��������������� f. E��F��������Ԫ�ع������Ӿ���
��5��
 ��������
��������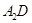 ,ԭ���� ��
,ԭ���� ����6��E���ʵľ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ����������ͼ����ʾ����E���ʵľ���ѻ�ģ��Ϊ ��
����֪Eԭ�Ӱ뾶Ϊr pm��
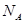 ��ʾ����٤�������������ı߳�Ϊa�������ĸ�Ϊh�������ܶȿɱ�ʾΪ g/cm3������ֻ��r��
��ʾ����٤�������������ı߳�Ϊa�������ĸ�Ϊh�������ܶȿɱ�ʾΪ g/cm3������ֻ��r��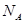 ����ʽ��ʾ��
����ʽ��ʾ��
��1�� ��2�֣�
��2�֣�
��2��sp2�ӻ�
��3��ֱ����
��4��abde
��5�����ݡ���������ԭ���������Է���NH3�����ڼ����ܼ�H2O��NH3������H2O���Ӽ�����γ������
��6���������ܶѻ� ��
�� 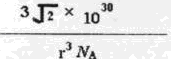
 ��2�֣�
��2�֣���2��sp2�ӻ�
��3��ֱ����
��4��abde
��5�����ݡ���������ԭ���������Է���NH3�����ڼ����ܼ�H2O��NH3������H2O���Ӽ�����γ������
��6���������ܶѻ�
 ��
�� 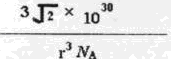
����������ɢ�֪H��Cu��D��OԪ�أ���A��HԪ�أ�BԪ��ԭ�Ӽ۵��ӣ���Χ���ӣ��Ų�ΪnSnnPn����n=2������B��CԪ�أ���C��NԪ�أ�FԪ��ԭ�ӵĺ���p����������s����������1,��F��Na��Al����������F��Na���ų�����F��AlԪ�أ�E��ԭ�Ӱ뾶��F��E�ĵ�һ������Ҳ��F��ͬ����Ԫ���б�ALԭ�Ӱ뾶���Ԫ����Na��Mg������Mg��3s���Ϊȫ��״̬����һ�����ܱ�Al������E��MgԪ�أ� G�Ļ�̬ԭ�Ӻ�����6��δ�ɶԵ��ӣ���G��CrԪ�أ���1��GԪ�ػ�̬ԭ�ӵļ۵����Ų�ʽΪ3d54s1���Ų�ͼΪ
 ��
����2�������ӻ�������ۣ�CO32-��Cԭ�ӵļ۲���Ӷ���=3+1/2(4+2-3��2)=3������Cԭ����sp2�ӻ���
��3��N2O��CO2�ǵȵ����壬����N2O��CO2�Ŀռ乹����ͬ������ֱ���ͷ��ӣ�
��4��E��F��ͬ����Ԫ�أ���F��ԭ������С��E��a��E��F�����ӵĵ��Ӳ�ṹ��ͬ���˵����������Ӱ뾶С���������Ӱ뾶E>F����ȷ��b��ͬ����Ԫ�غ˵������ĵ縺�Դ����Ե縺��F>E,��ȷ��c��Mg��ԭ�Ӱ뾶��Al������Al�Ľ�������ǿ���۵�ߣ�F>E������d��E��F�Ļ�ԭ�Խ�ǿ����������������û���Ӧ����Mg�������̼�ķ�Ӧ�����ȷ�Ӧ����ȷ��e��E���������������������ᷴӦ��������Ӧ���������ԣ���ȷ��f���Ȼ����Ƿ��Ӿ��壬����ѡabde��
��5�������Ǽ��Է��ӣ�ˮҲ�Ǽ��Է��ӣ����ݡ���������ԭ���������Է���NH3�����ڼ����ܼ�H2O����NH3������H2O���Ӽ�����γ������
��6��Mg�ľ����ṹ��ʾMg���������ܶѻ������ݡ���̯�������㾧����Mg��ԭ�Ӹ�����1+1/8��8=2,�����ı߳�a=2r����h=
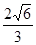 a,1pm=1��10-10cm��
a,1pm=1��10-10cm�������ܶ�=2��24/NA/
 a2h=
a2h= =
=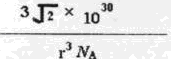

��ϰ��ϵ�д�
�����Ŀ