��Ŀ����
Cu3N�������õĵ�ѧ��ѧ���ܣ��ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ��ҵ�������У������Ź㷺�ġ���������ľ����á�
(1)��N3-������ͬ����������ԭ�ӷ��ӵĿռ乹���� ��
(2)Cu�������õĵ��硢���Ⱥ���չ�ԣ������Cu���е����Ե�ԭ�� ��
(3)��Cu�Ĵ������£��Ҵ��ɱ���������Ϊ��ȩ����ȩ������̼ԭ�ӵ��ӻ���ʽ��__________����ȩ������H��C��O�ļ���__________�Ҵ������е�H��C��O�ļ���(����ڡ��������ڡ���С�ڡ�)��
(4)Cu+�ĺ�������Ų�ʽΪ ������������Һ�в��ȶ����ɷ����绯��Ӧ����Cu2+��Cu����CuO�ڸ����»�ֽ��Cu2O���Դӽṹ�ǶȽ�������CuOΪ�λ�����Cu2O ��
(5)[Cu(H2O)4]2+Ϊƽ�������νṹ�����е�����H2O��Cl��ȡ�������ֲ�ͬ�Ľṹ���Ի���[Cu(H2O)2(Cl)2]���м��Եķ��ӵĽṹʽ ��
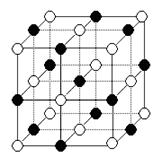
(6)Cu3N�ľ����ṹ��ͼ��ʾ��

N3������λ��Ϊ__________��Cu+�뾶Ϊapm��N3���뾶Ϊb pm��Cu3N���ܶ�__________g/cm3��(�����ӵ���Ϊ������NA��ʾ)
(1)��N3-������ͬ����������ԭ�ӷ��ӵĿռ乹���� ��
(2)Cu�������õĵ��硢���Ⱥ���չ�ԣ������Cu���е����Ե�ԭ�� ��
(3)��Cu�Ĵ������£��Ҵ��ɱ���������Ϊ��ȩ����ȩ������̼ԭ�ӵ��ӻ���ʽ��__________����ȩ������H��C��O�ļ���__________�Ҵ������е�H��C��O�ļ���(����ڡ��������ڡ���С�ڡ�)��
(4)Cu+�ĺ�������Ų�ʽΪ ������������Һ�в��ȶ����ɷ����绯��Ӧ����Cu2+��Cu����CuO�ڸ����»�ֽ��Cu2O���Դӽṹ�ǶȽ�������CuOΪ�λ�����Cu2O ��
(5)[Cu(H2O)4]2+Ϊƽ�������νṹ�����е�����H2O��Cl��ȡ�������ֲ�ͬ�Ľṹ���Ի���[Cu(H2O)2(Cl)2]���м��Եķ��ӵĽṹʽ ��
(6)Cu3N�ľ����ṹ��ͼ��ʾ��

N3������λ��Ϊ__________��Cu+�뾶Ϊapm��N3���뾶Ϊb pm��Cu3N���ܶ�__________g/cm3��(�����ӵ���Ϊ������NA��ʾ)
(1)V��
(2)CuΪ�������壬�����д��ڿ������ƶ��ĵ��ӣ�ͨ������ƶ���
(3)sp2 ��sp3������
(4)1s22s22p63s23p63d10 Cu+�۵���Ϊ3d10Ϊȫ�����ṹ���ȶ�
(5)

(6)6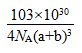
(2)CuΪ�������壬�����д��ڿ������ƶ��ĵ��ӣ�ͨ������ƶ���
(3)sp2 ��sp3������
(4)1s22s22p63s23p63d10 Cu+�۵���Ϊ3d10Ϊȫ�����ṹ���ȶ�
(5)

(6)6
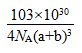
(1)N3-�ĵ�������10��������ͬ�ĵ�����������ԭ����H2O��Ӧ��V�ͷ��ӣ�
(2)��ΪCuΪ�������壬���ݡ������������ۣ������д��ڿ������ƶ��ĵ��ӣ�ͨ������ƶ���
(3)��ȩ�Ľṹ��ʽ��CH3CHO��ǰ��-CH3��C��sp3�ӻ���ȩ��-CHO�д���̼��˫�������е�̼ԭ�Ӳ�ȡ����sp2�ӻ���������120�㡣�Ҵ���Cԭ�Ӳ�ȡ����sp3�ӻ�������С��120�㣬���Լ��ǵĴ�С��ϵ����ȩ�����Ҵ���
(4)Cu+�ĺ�������Ų�ʽ��1s22s22p63s23p63d10��CuO�ڸ����»�ֽ�������ȶ���Cu2O������ΪCu+�۵���Ϊ3d10Ϊȫ�����ṹ���ȶ���
(5)[Cu(H2O)4]2+Ϊƽ�������νṹ��������ṹ��

(6)�۲쾧���Ľṹ֪�У������N3-(1/8)��Ӧ������������3��Cu+(1/4)����N3-��Cu+=1/8��3/4=1��6������λ����6�������������V=(2a+2b)3��10-30cm3������ͬ206/NA�����ܶ�Ϊ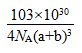 ��
��
(2)��ΪCuΪ�������壬���ݡ������������ۣ������д��ڿ������ƶ��ĵ��ӣ�ͨ������ƶ���
(3)��ȩ�Ľṹ��ʽ��CH3CHO��ǰ��-CH3��C��sp3�ӻ���ȩ��-CHO�д���̼��˫�������е�̼ԭ�Ӳ�ȡ����sp2�ӻ���������120�㡣�Ҵ���Cԭ�Ӳ�ȡ����sp3�ӻ�������С��120�㣬���Լ��ǵĴ�С��ϵ����ȩ�����Ҵ���
(4)Cu+�ĺ�������Ų�ʽ��1s22s22p63s23p63d10��CuO�ڸ����»�ֽ�������ȶ���Cu2O������ΪCu+�۵���Ϊ3d10Ϊȫ�����ṹ���ȶ���
(5)[Cu(H2O)4]2+Ϊƽ�������νṹ��������ṹ��

(6)�۲쾧���Ľṹ֪�У������N3-(1/8)��Ӧ������������3��Cu+(1/4)����N3-��Cu+=1/8��3/4=1��6������λ����6�������������V=(2a+2b)3��10-30cm3������ͬ206/NA�����ܶ�Ϊ
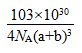 ��
��
��ϰ��ϵ�д�
�����Ŀ
 �ͺ�ɫ��HD���ֻ����
�ͺ�ɫ��HD���ֻ���� ��Bԭ�Ӳ�ȡ���ӻ��������Ϊ ��
��Bԭ�Ӳ�ȡ���ӻ��������Ϊ �� ���ӵĿռ乹��Ϊ ��
���ӵĿռ乹��Ϊ �� ��������
�������� ,ԭ���� ��
,ԭ���� �� ��ʾ����٤�������������ı߳�Ϊa�������ĸ�Ϊh�������ܶȿɱ�ʾΪ g/cm3������ֻ��r��
��ʾ����٤�������������ı߳�Ϊa�������ĸ�Ϊh�������ܶȿɱ�ʾΪ g/cm3������ֻ��r��
 ����________mol��
����________mol��