��Ŀ����
18��ijʵ��С����CO��NH2��2��������30% NaOH��Һ��NaHCO3�����Ʊ�ˮ���£�N2H4•H2O�������ⶨ�京������������ʵ�飺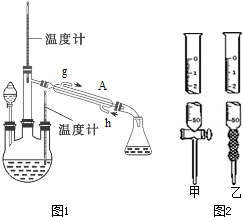
���Ʊ�NaClO��Һ��������ͨ��30% NaOH����������Һ�У���ַ�Ӧ��
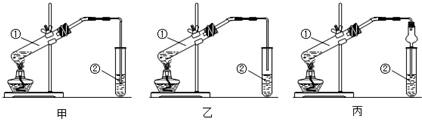
����ȡˮ���£���CO��NH2��2�������������ƿ�У��ٵ���Һ�����Һ©���У����Ʒ�Ӧ�¶ȣ�ʹ��Һ��������������ƿ�У���ַ�Ӧ����������������ƿ�ڵ���Һ���ռ���Ʒ��ʵ��װ����ͼ1ʾ��
�۲ⶨ��Ʒ��ˮ���µĺ�������ȡ���a g������������NaHCO3���壬��ˮ���250mL��Һ��ȡ��25.00mL����b mol•L-1��I2��Һ�ζ���ʵ��������I2��Һ��ƽ��ֵΪc mL������֪��N2H4•H2O+2I2�TN2��+4HI+H2O�����ش��������⣺
��1��װ��A�������������ܣ����ˮ��Ϊh���g����h������
��2���Ʊ�ˮ���µ�ԭ����CO��NH2��2+2NaOH+NaClO=Na2CO3+N2H4•H2O+NaCl���û�ѧ����ʽ��ʾ����
��3���ζ������У���Һ��pH�ܱ�����6.5���ҵ�ԭ����NaHCO3����ζ������в�����HI��Ӧ��
��4��I2��ҺӦ������ͼ2��ʾ�����ף���ס����ҡ����У��ζ�ʱ���õ�ָʾ��Ϊ���ۣ��ζ��յ����������Һ����ɫ����ɫ��
��5����Ʒ��ˮ���º����������������ı���ʽΪ$\frac{20bc��1{0}^{-3}}{a}$��100%��Ҫ���ʾ��������̣���Ҫд������������
��6���ζ������У����ζ����ڵζ�ǰ���첿�������ݣ��ζ�����ʧ����ⶨ�����ƫ�ߣ��ƫ�ߡ���ƫ�͡�����
���� ��1������װ��ͼ��������������ˮ�������������������෴ʱ����Ч���ã�
��2��CO��NH2��2��NaClO��NaOH��Ӧ����N2H4•H2O��NaCl��̼���ƣ����ԭ���غ�͵�ʧ�����غ���ƽ����ʽ��
��3�����ݵζ������ķ�Ӧ��֪����HI��NaHCO3���������ɵ�HI��
��4����ˮ�������Ժ������ԣ�Ӧ��ѡ����ʽ�ζ���ʢ�ţ��õⵥ�ʽ��еζ�ʵ��ʱ�����õ�����ָʾ�����ﵽ�յ㣬�ⵥ�ʹ�����Һ����ɫ��
��5���������ĵĵ����������ʵ������ɷ���ʽ����N2H4•H2O�����ʵ������������ټ��㺬����
��6���ζ����ڵζ�ǰ���첿�������ݣ��ζ�����ʧ�������ĵĵ���Һ�����ƫ��
��� �⣺��1������װ��ͼ��֪AΪ�����ܣ�������������ˮ�������������������෴ʱ����Ч���ã���������ˮ��h�ڽ��룬��g��������
�ʴ�Ϊ�������ܣ�h��
��2��CO��NH2��2��NaClO��NaOH��Ӧ����N2H4•H2O��NaCl��̼���ƣ���Ӧ��CԪ��ʧ���ӣ�ClԪ�صõ��ӣ���÷�Ӧ�ķ���ʽΪCO��NH2��2+2NaOH+NaClO=Na2CO3+N2H4•H2O+NaCl��
�ʴ�Ϊ��CO��NH2��2+2NaOH+NaClO=Na2CO3+N2H4•H2O+NaCl��
��3���ζ������ķ�ӦΪN2H4•H2O+2I2�TN2��+4HI+H2O���ζ�����������HI������NaHCO3���������ɵ�HI��������Һ��pH�ܱ�����6.5���ң�
�ʴ�Ϊ��NaHCO3����ζ������в�����HI��Ӧ��
��4����ˮ�������Ժ������ԣ�Ӧ��ѡ����ʽ�ζ���ʢ�ţ���ѡ�������ף����ʵ��������ۻ����ɫ�������õⵥ�ʽ��еζ�ʵ��ʱ�����õ�����ָʾ�����ζ�ǰ����ƿ����ҺΪN2H4•H2O���ﵽ�յ�ʱ��N2H4•H2O����ȫ���ģ��ⵥ�ʹ�������Һ����ɫ��
�ʴ�Ϊ���ף����ۣ���Һ����ɫ����ɫ��
��5����֪b mol•L-1��I2��Һ�ζ���ʵ��������I2��Һ��ƽ��ֵΪc mL����n��I2��=b mol•L-1��c��10-3L=bc��10-3mol����֪��N2H4•H2O+2I2�TN2��+4HI+H2O��
��n��N2H4•H2O��=$\frac{1}{2}$n��I2��=$\frac{bc}{2}$��10-3mol�����Բ�Ʒ��ˮ���º���������������=$\frac{\frac{bc}{2}��1{0}^{-3}��40g}{ag}$��100%=$\frac{20bc��1{0}^{-3}}{a}$��100%��
�ʴ�Ϊ��$\frac{20bc��1{0}^{-3}}{a}$��100%��
��6���ζ����ڵζ�ǰ���첿�������ݣ��ζ�����ʧ�������ĵĵ���Һ�����ƫ���ɷ���ʽ��֪n��N2H4•H2O��=$\frac{1}{2}$n��I2������N2H4•H2O�����ʵ���ƫ�����Լ������N2H4•H2O������ƫ���ⶨ���ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
���� ���⿼�������ʺ����IJⶨ�����ʵ��Ʊ�����Ŀ�漰����ʵ���������Ӧ����ʽ����д���ζ�ԭ����Ӧ�á����ݴ����ȣ���Ŀ�Ѷ��еȣ������ڿ���ѧ����ʵ�������ͼ���������

| A�� | �ҹ�����ġ��϶����š�������ʹ�õ�̼��ά����һ���������ǽ������� | |
| B�� | ֻҪ������������ʳ��ɫ�ء�����Ԫ�ء������������Ρ�������ΪijЩʳƷ�����Ӽ� | |
| C�� | ���������ܽ���ˮ������������Һ�����ж����ЧӦ | |
| D�� | ���ع��͡������ӹ��������������Ʒ������������ |
| A�� | �������Ƶĵ���ʽ��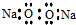 | |
| B�� | ������Ϊ35��������Ϊ45����ԭ�ӣ�${\;}_{35}^{80}Br$ | |
| C�� | �����ӵĽṹʾ��ͼ��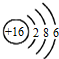 | |
| D�� | NH3 �ĵ���ʽ��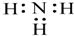 |
| A�� | $\frac{a+b}{4}$mol | B�� | $\frac{a+c}{4}$mol | C�� | $\frac{3a+c}{4}$mol | D�� | cmol��$\frac{b}{3}$mol |
 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��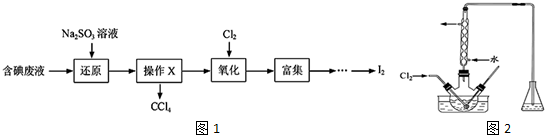
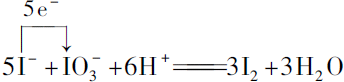 ��
��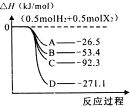 ±�صĵ��ʼ��仯������������������Ӧ�ù㷺��
±�صĵ��ʼ��仯������������������Ӧ�ù㷺�� ����${\;}_{1}^{2}$H����
����${\;}_{1}^{2}$H����