��Ŀ����
6��I��ʵ���ҴӺ����Һ����H2O�⣬����CCl4��I2��I-�ȣ��л��յ⣬��ʵ��������£�
��1�����Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI-�������ӷ���ʽΪSO32-+I2+H2O=2I-+SO42-+2H+���ò�����I2��ԭΪI-��Ŀ����ʹCCl4�еĵ����ˮ�㣮
��2������X������Ϊ��Һ��
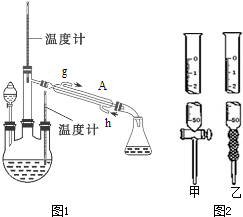
��3������ʱ��������ƿ�н���I-��ˮ��Һ���������pHԼΪ2������ͨ��Cl2����400C���ҷ�Ӧ��ʵ��װ����ͼ��ʾ����ʵ������ڽϵ��¶��½��е�ԭ����ʹ��������Һ���нϴ���ܽ�ȣ����ֹ���������ֹ���һ��������������ƿ��ʢ�ŵ���ҺΪNaOH��Һ��
���û�ѧ�����ⶨ���⻯��ʱ���������á���ѧ�Ŵ�Ӧ����������Ŵ�Ȼ���ٽ��вⶨ�������ǡ���ѧ�Ŵ�Ӧ��ʵ�鲽�裺
������I-���ҳ����Ի���������Һ�������ˮ����I-��ȫ������IO3-�����ȥ��������Br2��
�����ɢ��Ƶõ�ˮ��Һ�м������������KI��Һ����ʹ��Ӧ������ȫ��
���ڢڵõ���ˮ��Һ�м���������CCl4���������ɵ�I2��ˮ��Һ��ȫ��ת�Ƶ�CCl4 �У��÷�Һ©����Һȥ��ˮ�㣻
����۵õ���CCl4������£�������H2N-NH2����ˮ��Һ����ʹI2��ȫ��I-��ʽ��CCl4�����ˮ�㣬�÷�Һ©����Һȥ��CCl4�㣮
���������IJ��õ���ˮ��Һ�����ͨ����Ӧ�����Ŵ��˵ĵ⣬��ش��������⣺
��1��д������ڵ����ӷ���ʽ����������з�Ӧ�ĵ���ת�Ƶķ������Ŀ��
 ��
����2��������ʵ������г��ֵ���ʧ������һ�Ρ���ѧ�Ŵ���Һ�I-������ԭ��Һ��I-������6��������n�Ρ���ѧ�Ŵ���Һ�I-������ԭ��Һ�������6n����
���� I�����Һ������������Һ���ѵ��ʵԭΪI-�����Ȼ�̼������ˮ����ֲ㣬���Һ���ɵõ����Ȼ�̼��ʣ�����Һ�м���������I-�õ�I2�����������õ��ϴ���I2��
��1������������ԣ������������������������ƣ���������ԭ���ɵ⣻������ˮ����������������ˮ��
��2�����뻥�����ܵ�Һ����÷�Һ�ķ������룻
��3���������������������ܽ�������¶ȵ����߶���С�����������������ܺ�����������Һ��Ӧ���������ʣ�
II����1�������������£�I-��IO3-��Ӧ����I2��ˮ��I-ʧ���ӣ�IO3-�õ��ӣ��ݴ˷�����
��2���ٵ�һ����I-+3Br2+3H2O=6Br-+IO3-+6H+��
�ڵڶ�����IO3-+5I-+6H+=3I2+3H2O��
�ܵ��IJ���N2H4+2I2=4I-+N2��+4H+��
���ݷ�Ӧ��֮������ʵ�����ϵ�жϣ�
��� �⣺I�����Һ������������Һ���ѵ��ʵԭΪI-�����Ȼ�̼������ˮ����ֲ㣬���Һ���ɵõ����Ȼ�̼��ʣ�����Һ�м���������I-�õ�I2�����������õ��ϴ���I2��
��1������������ԣ������������������������ƣ���������ԭ���ɵ����ӣ����ӷ�Ӧ����ʽΪSO32-+I2+H2O=2I-+2H++SO42-��
������ˮ����������������ˮ��Ϊ��ʹ�����IԪ�ؽ���ˮ��ҺӦ���ԭΪ�����ӣ�
�ʴ�Ϊ��SO32-+I2+H2O=2I-+2H++SO42-��ʹCCl4�еĵ����ˮ�㣻
��2�����Ȼ�̼������ˮ������ֲ㣬���뻥�����ܵ�Һ����÷�Һ�ķ������룬���Է�������Ȼ�̼���÷�Һ�ķ������ʴ�Ϊ����Һ��
��3���������������������ܽ�������¶ȵ����߶���С���¶�Խ�ߣ��������ܽ��ԽС����ӦԽ����֣�����Ӧ���ڵ��������½��з�Ӧ�����������������ж�������ֱ���ſգ��Ҷ��ܺ�����������Һ��Ӧ���������ʣ�������NaOH��Һ���������͵�������
�ʴ�Ϊ��ʹ��������Һ���нϴ���ܽ�ȣ����ֹ���������ֹ���һ������������NaOH��Һ��
II����1�������������£�I-��IO3-��Ӧ����I2��ˮ��I-ʧ���ӣ�IO3-�õ��ӣ���Ӧ�ĵ���ת�Ƶķ������ĿΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2���ٵ�һ����I-+3Br2+3H2O=6Br-+IO3-+6H+��
�ڵڶ�����IO3-+5I-+6H+=3I2+3H2O��
�ܵ��IJ���N2H4+2I2=4I-+N2��+4H+��
��˴����Ϸ���ʽ�ͷ����ɵù�ϵʽ��I-��IO3-��3I2��6I-
1mol 6mol
��һ�Ρ���ѧ�Ŵ���Һ�I-������ԭ��Һ��I-������6����
���پ���һ�Ρ���ѧ�Ŵ�I-��IO3-��3I2��6I-
6mol 36mol
�����Ρ���ѧ�Ŵ���Һ�I-������ԭ��Һ��I-������36����
���Ծ���n�Ρ���ѧ�Ŵ���Һ�I-������ԭ��Һ�������6n����
�ʴ�Ϊ��6��6n��
���� ���⿼�������ʵķ����ᴿʵ�鷽����ƣ���ȷ���ʵ������ǽⱾ��ؼ����������ʵ��������ʡ�����������ᴿ������ѡȡ�ȷ������������֪����ļ��鷽������Ŀ�Ѷ��еȣ�

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д�| A�� | �ǽ���Ԫ��ԭ����ɵĻ����ﲻ���������ӻ����� | |
| B�� | ��A��Ԫ�صĽ�����һ���Ȣ�A��Ԫ�صĽ�����ǿ | |
| C�� | ͬһ����Ԫ����ɵĻ�����һ���ǹ��ۻ����� | |
| D�� | NH5�е�����ԭ�ӵ�����㶼������Ӧϡ������ԭ�ӵ��Ӳ�ṹ��1 mol NH5�к���4NA��N-H����NA��ʾ�����ӵ�������ֵ�� |
| A�� | ����������Һ���������Һ��һ��������Ϻ������ᾧ���� | |
| B�� | ����Һ�����������ӿ��Դ������棺H+��SO42-��I-��C6H5OH | |
| C�� | ����Һ������Ũ�ȴ�С��ϵΪ��SO42-��Fe3+��H+��OH- | |
| D�� | ����0.1 molNH4Fe��SO4��2��Һ�еμ�0.1 molBa��OH��2����Ӧ�����ӷ���ʽΪ��Fe3++2SO42-+2Ba2++3OH-=2BaSO4��+Fe��OH��3�� |
| A�� | 10��20 mL 3mol/L��X��Һ | B�� | 20��30 mL 2molL��X��Һ | ||
| C�� | 20��10 mL 4mol/L��X��Һ | D�� | 10��10 mL 2mol/L��X��Һ |
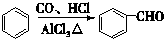
 ��
�� +2Cu��OH��2$\stackrel{��}{��}$
+2Cu��OH��2$\stackrel{��}{��}$ +Cu2O��+2H2O��
+Cu2O��+2H2O�� $\stackrel{һ��������}{��}$
$\stackrel{һ��������}{��}$ +nH2O��
+nH2O�� ��
��
 �����ᣨH3PO2����һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԣ��ش��������⣺
�����ᣨH3PO2����һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԣ��ش��������⣺