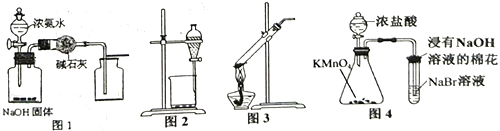
��Ŀ����
����Ŀ��ҩ���м���Q��ҽ�ò���PVA�ĺϳ�·�����¡�

��֪��
��1��A�ķ���ʽ��C6H6��A��B�ķ�Ӧ������_______��
��2��B��C��������Ӧ���Լ�a��_______��
��3��C��DΪȡ����Ӧ���仯ѧ����ʽ��_______��
��4��F���еĹ�������________��
��5��F�ĺ���̼̼˫����������ͬ���칹����_____�֣���˳���칹�壬����F�������к˴Ź���������3�����շ壬���ܷ���������Ӧ�Ľṹ��ʽ��___��
��6��G��X�Ļ�ѧ����ʽ��________��
��7��W�ܷ����ۺϷ�Ӧ���γɵĸ߷��ӽṹ��ʽ��________��
��8��������E+W��Q������ͼ���������������߿���д�����ʵĽṹ��ʽ����_________

���𰸡�ȡ����Ӧ Ũ���ᡢŨ���� 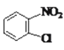 +2NH3
+2NH3![]()
![]() +NH4Cl ̼̼˫�������� 5 CH2=C��CH3��OOCH
+NH4Cl ̼̼˫�������� 5 CH2=C��CH3��OOCH 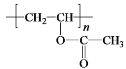 +nH2O
+nH2O![]()
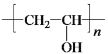 +NCH3COOH
+NCH3COOH 
 ��
��
��������
����Q�ṹ��ʽ��A����ʽ֪��AΪ![]() ��A����ȡ����Ӧ����B��BΪ�ȱ���B��C��������Ӧ����C�к�����ԭ�Ӻ�������D������ԭ��Ӧ����E������Q�ṹ��ʽ֪��E�к����������ڵİ�������EΪ
��A����ȡ����Ӧ����B��BΪ�ȱ���B��C��������Ӧ����C�к�����ԭ�Ӻ�������D������ԭ��Ӧ����E������Q�ṹ��ʽ֪��E�к����������ڵİ�������EΪ ��DΪ
��DΪ![]() ��CΪ
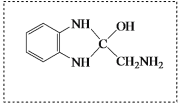
��CΪ![]() ���Լ�aΪŨ���ᡢŨ���W�ܷ����ۺϷ�Ӧ�����Q��E�ṹ��ʽ֪��WΪH2NCH2COOH��Y����ȡ����Ӧ����W��X����ȡ����Ӧ����Y��YΪClCH2COOH��XΪCH3COOH��Gˮ������X��PVA����GΪ
���Լ�aΪŨ���ᡢŨ���W�ܷ����ۺϷ�Ӧ�����Q��E�ṹ��ʽ֪��WΪH2NCH2COOH��Y����ȡ����Ӧ����W��X����ȡ����Ӧ����Y��YΪClCH2COOH��XΪCH3COOH��Gˮ������X��PVA����GΪ![]() ��FΪCH3COOCH=CH2��E��W�ȷ���ȡ����Ӧ����
��FΪCH3COOCH=CH2��E��W�ȷ���ȡ����Ӧ����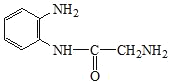 ��Ȼ������Ϣ�еļӳɷ�Ӧ����
��Ȼ������Ϣ�еļӳɷ�Ӧ���� ��
��
��1��AΪ����BΪ�ȱ���A��B�ķ�Ӧ������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
��2��B��C��������Ӧ���Լ�a��Ũ���ᡢŨ���ᣬŨ�������������ʴ�Ϊ��Ũ���ᡢŨ���
��3��C��DΪȡ����Ӧ,�仯ѧ����ʽ��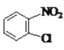 +2NH3
+2NH3![]()
![]() +NH4Cl���ʴ�Ϊ��
+NH4Cl���ʴ�Ϊ�� +2NH3
+2NH3![]()
![]() +NH4Cl��
+NH4Cl��
��4��FΪCH3COOCH=CH2��F���еĹ�������̼̼˫�����������ʴ�Ϊ��̼̼˫����������
��5������ʽ����CH4H6O2�Һ���̼̼˫���������Ľṹ�У�CH3COOCH=CH2(��˳���칹)��HCOOCH2CH=CH2(��˳���칹)��HCOOC(CH3)=CH2(��˳���칹)��HCOOCH=CHCH3(˳ʽ����ʽ���ֽṹ)��CH2=CHCOOCH3(��˳���칹)����6�֣���ȥF����5�֣����к˴Ź���������3�����շ壬���ܷ���������Ӧ�ĽṹΪ��3�����Һ���ȩ�����ṹ��ʽΪCH2=C��CH3��OOCH���ʴ�Ϊ��5��CH2=C��CH3��OOCH��
��6��G��X�Ļ�ѧ����ʽ��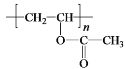 +nH2O
+nH2O![]()
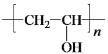 +NCH3COOH���ʴ�Ϊ��
+NCH3COOH���ʴ�Ϊ�� +nH2O
+nH2O![]()
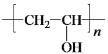 +NCH3COOH��
+NCH3COOH��
��7��W�ܷ����ۺϷ�Ӧ,�γɵĸ߷��ӽṹ��ʽ��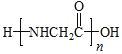 ���ʴ�Ϊ��
���ʴ�Ϊ��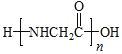 ��
��
��8��E��W�ȷ���ȡ����Ӧ���� ��Ȼ������Ϣ�еļӳɷ�Ӧ����
��Ȼ������Ϣ�еļӳɷ�Ӧ���� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����仯�����ڹ�ҵ��ҽҩ��ũҵ�Ȳ�����������;��ij����������(��Ҫ�ɷ�ΪB2O3��2MgO������SiO2��CaO��FeO������)��ȡ�����ᡢ����þ�Ĺ�������ͼΪ��
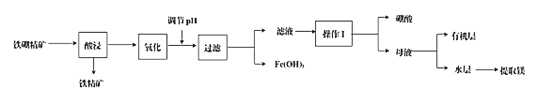
��֪�������ڲ�ͬ�¶��µ��ܽ�ȣ�
�¶�(��) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
�ܽ��(g/100gˮ) | 3 | 3 | 5 | 7 | 9 | 11 | 15 | 18 | 23 | 29 |
�ش��������⣺
(1)ʹ�������������������Ҫ��Ӧ�Ļ�ѧ����ʽΪ_________��Ϊ��߽������ʣ��ɲ�ȡ�Ĵ�ʩ��_________(д������)��
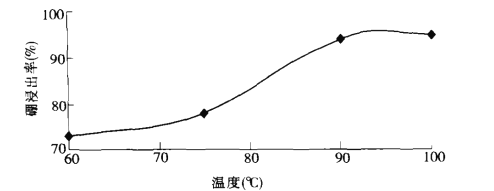
(2)���ʱ���¶���������ʵĹ�ϵ��ͼ��ʾ������ʵ�����¶�Ϊ_________��
(3)����Һ�����������ǽ���Һ�е�Fe2+��_______�Լ�����ΪFe3+����Ӧ�����ӷ���ʽΪ_________��
(4)����Һ�л��H3BO3�����������I�����������_________��
(5)����Һ�м����л���ȡ����ȡ��Һ����ʱ���ᴦ��_________����(�����л�����������)��ʵ����ģ����ȡ����ʹ�õIJ����������ձ��⣬��һ��Ҫ����������_________��
(6)ij������ m1 kg�������Ʊ����ᣬ�õ�����Ϊ99.8%������m2 kg�����������������������__________(��ʽ����)��