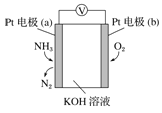
题目内容
【题目】原子序数依次增大的X、Y、Z、Q、E五种元素中,X元素原子核外有三种不同的能级且各个能级所填充的电子数相同,Z是地壳内含量(质量分数)最高的元素,Q原子核外的M层中只有两对成对电子,E元素原子序数为29。
用元素符号或化学式回答下列问题:
(1)Y在周期表中的位置为__________________。
(2)已知YZ2+与XO2互为等电子体,则1mol YZ2+中含有π键数目为___________。
(3)X、Z与氢元素可形成化合物XH2Z,XH2Z分子中X的杂化方式为_________________。
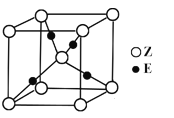
(4)E原子的核外电子排布式为__________;E有可变价态,它的某价态的离子与Z的阴离子形成晶体的晶胞如图所示,该价态的化学式为____________。
(5)氧元素和钠元素能够形成化合物F,其晶胞结构如图所示(立方体晶胞),晶体的密度为ρg··cm-3,列式计算晶胞的边长为a=______________cm(要求列代数式)。

【答案】 第二周期 第VA族 2NA或1.204×1024 sp2杂化 1s22s22p63s13p23d63d104s1或[Ar]3d104s1 Cu2O 
【解析】X元素原子核外有三种不同的能级且各个能级所填充的电子数相同,则X为C,Z是地壳中含有最高的元素,即Z为O,,因为原子序数依次增大,则Y为N,Q原子核外的M层中只有两对成对电子,即Q为S,E的原子序数为29,则E为Cu,(1)考查元素在元素周期表中的位置,Y是N,位于第二周期VA族;(2)考查等电子体和π键判断,YZ2+为NO2+,与CO2互为等电子体,等电子体的结构相似,CO2的结构式为O=C=O,因此1molNO2+中含有π键的数目为2NA个;(3)考查杂化类型,形成的化合物是HCHO,其中碳原子是sp2杂化;(4)考查核外电子排布式,通过晶胞的结构确定化学式,Cu位于第四周期IB族,核外电子排布式为1s22s22p63s13p23d63d104s1或[Ar]3d104s1 ;O原子位于顶点和体心,个数为8×1/8+1=2,Cu全部位于体心,因此化学式为Cu2O;(5)考查晶胞的计算,核外电子排布相同时,半径随着原子序数增大而减小,即氧元素位于顶点和面心,个数为8×1/8+6×1/2=4,Na元素位于晶胞内,有8个,因此化学式为Na2O,晶胞的质量为4×62/NAg,晶胞的体积为a3cm3,根据密度的定义,有ρ=4×62/(NA×a3),即边长为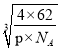 cm。
cm。

 53随堂测系列答案
53随堂测系列答案【题目】碘及其化合物在科研、生活等方面有广泛用途。回答下列问题:
(1)海带中富含碘,按如下实验流程可对海带中碘的含量进行测定。

取0.0100 mol/L的AgNO3标准溶液装入棕色滴定管,取100.00 mL海带浸取原液至滴定池,用电势滴定法测定碘含量。测得的电动势(E) 反映溶液中c(Iˉ)的变化,部分数据如下表:
V(AgNO3)/mL | 15.00 | 19.00 | 19.80 | 19.98 | 20.00 | 20.02 | 21.00 | 23.00 | 25.00 |
E/mV | -225 | -200 | -150 | -100 | 50.0 | 175 | 275 | 300 | 325 |
①灼烧海带时,除需要坩埚外,还需要用到的实验仪器是____________ (填序号)。
a.烧杯 b.三脚架 c.温度计 d.泥三角 e.酒精灯 f.坩埚钳
②使用棕色滴定管的原因是_____________________。
③根据表中数据,计算海带中碘的百分含量为____________。
(2)“大象的牙膏”是著名化学实验之一,其实验方法是将浓缩的过氧化氢溶液与肥皂液混合,再滴加少量碘化钾溶液,即可观察到泡沫状物质像喷泉一样喷涌而出。
已知:2H2O2(l)=2H2O(l)+O2(g) △H=-196kJ/mol,活化能Ea=76kJ/mol,若用I-催化时活化能Ea’=57kJ/mol。
①在H2O2溶液中加入KI溶液作催化剂,反应过程中发生I-与IO-之间的转化,请依次写出发生反应的离子方程式:
反应甲:______________________;
反应乙:______________________。
②反应甲为吸热反应,且甲的反应速率小于乙的反应速率,在下图中画出在H2O2溶液中加入KI后,反应过程的能量变化图。_______

(3)HI不稳定,其水溶液具有强酸性。现用0.lmol/LKI溶液、NH4I固体、pH试纸设计实验验证上述性质。简述实验方案。
①强酸性:__________________________。
②不稳定性:___________________________。