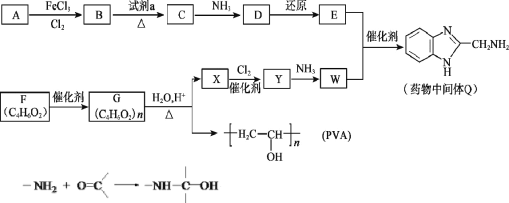
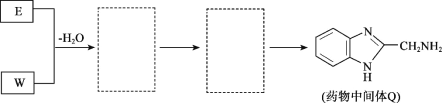
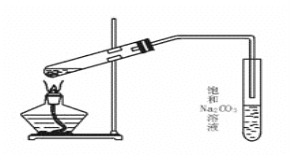
��Ŀ����
����Ŀ�����������(LiPF6)��������ˮ�������ڴ����л��ܼ�����������ӵ�صĵ���ʡ�ij������ʯ[Ca5(PO4)3F]Ϊ��Ҫԭ�ϣ��Ʊ���������﮵��������£�
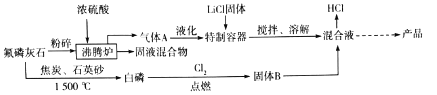
��֪��HF���۵�Ϊ-83�����е�Ϊ19.5��
�ش��������⣺
(1)�������ʯ��Ŀ����________________________
(2)�������������ò���������ԭ����________________(����������)
(3)����¯�в�����Һ�����û�����к���CaSO4��________(��������)
(4)�Ʊ�����(P4)�в���SiF4��һ�ֻ�ԭ�����壬�Ʊ����Ļ�ѧ����ʽΪ________________
(5)β����PCl5���������ռ���Һ�������������Σ�д��������Ӧ�����ӷ���ʽ��________________
(6)���42.5kgLiCl���뷴Ӧ�������Ͽ��Ʊ�________kgLiPF6
���𰸡�����Ӵ�������ӿ췴Ӧ���� HF���벣�����մ������еĶ������跴Ӧ H3PO4 4Ca5(PO4)3F+21SiO2+30C![]() 20CuSiO3+3P4+SiF4��+30CO�� PCl5+8OH-=PO43-+5Cl-+4H2O 152
20CuSiO3+3P4+SiF4��+30CO�� PCl5+8OH-=PO43-+5Cl-+4H2O 152
��������
����ʯ�������Ũ������ȵ������·���Ca5[PO4]3F+5H2SO4=HF��+3H3PO4+5CaSO4������AΪHF��Һ����HF����������跴Ӧ�������ڲ��������з�Ӧ������������������LiCl��Ӧ������ʯ�뽹̿��ʯӢɰ��1500������4Ca5(PO4)3F+21SiO2+30C![]() 20CuSiO3+3P4+SiF4��+30CO����������������ȼ���������������Ȼ��������Ȼ��Ļ�������LiCl��Ӧ����LiPF6��HCl��
20CuSiO3+3P4+SiF4��+30CO����������������ȼ���������������Ȼ��������Ȼ��Ļ�������LiCl��Ӧ����LiPF6��HCl��
(1)�������ʯ�����¹������С���Ӵ����������Ŀ��Ϊ����Ӵ�������ӿ췴Ӧ���ʣ�
(2)���ɵ�����ΪHF��HF���벣�����մ������еĶ������跴Ӧ������������������Ӧ��
(3)���ݷ�Ӧ�ķ���ʽ��������к���CaSO4��H3PO4��
(4) �Ʊ�����(P4)�в���SiF4��һ�ֻ�ԭ������CO����Ӧ�ķ���ʽΪ4Ca5(PO4)3F+21SiO2+30C![]() 20CuSiO3+3P4+SiF4��+30CO����
20CuSiO3+3P4+SiF4��+30CO����
(5)β����PCl5���������ռ���Һ�������������ƺ��Ȼ��ƣ����ӷ���ʽΪPCl5+8OH-=PO43-+5Cl-+4H2O��
(6)LiCl+6HF+PCl5=LiPF6+6HCl��42.5kgLiCl�����ʵ���Ϊ1000mol����������1000molLiPF6������Ϊ152kg��

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�����Ŀ��������ͭ(Cu2O)�������մɡ��������������������з�Ӧ�Ʊ���2(CuSO4H2O)![]() Cu2O+SO2��+SO3��+O2��+10H2O���ش��������⣺
Cu2O+SO2��+SO3��+O2��+10H2O���ش��������⣺
(1)Cu2O�л�̬ͭ�����ӵļ۲�����Ų�ͼΪ________________��������Ӧ�У��縺������Ԫ����________________����Ԫ�ط���)
(2)Ԫ��Cu��Ni���ڣ����һ�������������������ʾ��
I1/(kJ/mol) | I2/(kJ/mol) | |
Ni | 737.1 | 1753.0 |
Cu | 745.5 | 1957.9 |
ͭ�ĵ�һ���������ܶ�������Ӧ�ĸߣ���ԭ�����ͭ�ĺ˵�����ȶ࣬������ӵ����������⣬����һ��ԭ����________��
(3)��֪H2SO4�Ľṹʽ��ͼ��ʾ,������Ӧ�У�

�������������У�VSEPRģ����SO42-��ͬ����________(�ѧʽ)
��SO3���ӵ����幹����________________��д��һ����SO3��Ϊ�ȵ���������ӣ�________�������ӷ���)
(4)Cu2+��Cu+��CN-��OH-��NH3��H2O����ɶ��������ӡ�[Cu(NH3)4]2+�е�Cu2+��λ��Ϊ________��
(5)ͭԪ�غ���Ԫ���һ�־���M��M�ľ�����ͼ��ʾ��

��֪��NA�ǰ����ӵ�������ֵ��M�����ܶ�Ϊdgcm-3��M�ľ�����Cu+��Cl-������Ϊ________����������Ϊ________pm(�ú�d��NA�Ĵ���ʽ��ʾ)��(�ú�d��NA�Ĵ���ʽ��ʾ)
����Ŀ����E��F�����ܱ�������,��һ�������·�����Ӧ��E(g)��F(s)![]() 2G(g)�����Թ��������ƽ��ʱG���������(%)���¶Ⱥ�ѹǿ�ı仯���±���ʾ��
2G(g)�����Թ��������ƽ��ʱG���������(%)���¶Ⱥ�ѹǿ�ı仯���±���ʾ��
ѹǿ/MPa �������/% �¶�/�� | 1.0 | 2.0 | 3.0 |
810 | 54.0 | a | b |
915 | c | 75.0 | d |
1000 | e | f | 83.0 |
��b��f ��915�桢2.0MPaʱE��ת����Ϊ60%���۸÷�Ӧ����S��0 ��K(1000��)��K(810��)
�����١�������ȷ����( )
A.4��B.3��C.2��D.1��