��Ŀ����
����Ŀ���⻯��������״���������μ�����̵����������ȡ�ʵ������NaOH�����ʵ��ˮ����(N2H4��H2O)Ϊԭ�Ͽ��Ʊ��⻯�ơ�
������ʾ��ˮ�����л�ԭ�ԣ�������ˮ���ܽ��������NaIO3��һ����������
�ش��������⣺
��1��ˮ���µ��Ʊ����йط�Ӧԭ��Ϊ��NaClO + 2NH3 = N2H4��H2O + NaCl��
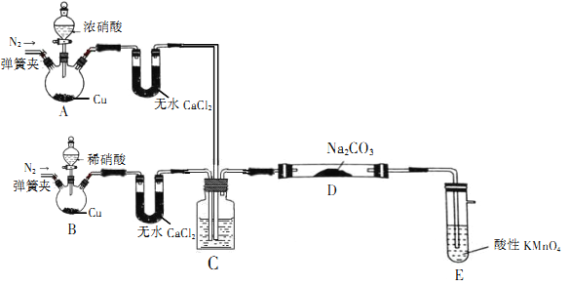
������ͼװ����ȡˮ���£�������˳��Ϊ_________(������������Сд��ĸ��ʾ)��




��װ��A��������_______��
�ۿ�ʼʵ��ʱ�������������еμ�Ũ��ˮ��һ��ʱ�������B��������ƿ�еμ�NaClO��Һ���μ�NaClO��Һʱ���ܹ��������___________��
��2���⻯�Ƶ��Ʊ�
��.��������ƿ�м���8.4gNaOH��30mLˮ�����衢��ȴ������25.4g�ⵥ�ʣ���������������������60~70������Ӧ��֣�
�������������Թ�����N2H4��H2O(ˮ����)����ԭNaIO��NaIO3����NaI��Һ��Ʒ��ͬʱ�ͷ�һ�ֿ����е����壻
������������ӦҺ�м���1.0g����̿����а�Сʱ��Ȼ����Һ�����̿���룻
���������袣���������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ò�Ʒ24.0g��
�ܲ��袡���˲��õļ��ȷ�����ˮԡ���ȣ� �ò��跴Ӧ��ȫ��������_________�����袢��IO3�����뷴Ӧ�����ӷ���ʽΪ________________________________��
�ݲ��袣 ������Һ�����̿���롱�ķ����dz��ȹ��ˡ�
�ޱ���ʵ�����Ϊ__________��ʵ�鷢�֣�ˮ����ʵ������������ֵƫ�ߣ����ܵ�ԭ����___________��
��ijͬѧ�����ƷNaI���Ƿ����NaIO3���ʡ�ȡ����������Ʒ���Թ��У���ˮ�ܽ⣬�μ���������Һ���ٵμ�����ϡ���ᣬƬ�̺���Һ�������ó�NaI�к���NaIO3���ʡ������۸�ʵ����۵ĺ�����____________��������Ϊ����д�����ӷ���ʽ������Ϊ������˵�����ɣ�
���𰸡�f a b c d e (ab˳��ɻ���) ��ֹ������ȫƿ ����μ�NaClO��Һ��������NaClO��Һ����ˮ���£����Ͳ��� �����������Һ����ɫ 2IO3��+ 3N2H4��H2O =3N2��+2I-+9H2O 80% ˮ��������ˮ�е��ܽ�����Ӧ ������I�������Ի����б�O2������I2��ʹ���۱���
��������
(1)���ɷ�Ӧԭ��NaClO+2NH3=N2H4![]() H2O+NaCl��֪,����Dװ�ò�������,Ϊ��ֹ��������,�������װ��A��,Ȼ����ͨ��c���ܽ���װ��B��,Ȼ����������백��Ӧ,����İ����Cװ������;������˳��Ϊfabcde;��ȷ��:fabcde����װ��A�������Ƿ�ֹ������ȫƿ;��ȷ��:��ֹ������ȫƿ��
H2O+NaCl��֪,����Dװ�ò�������,Ϊ��ֹ��������,�������װ��A��,Ȼ����ͨ��c���ܽ���װ��B��,Ȼ����������백��Ӧ,����İ����Cװ������;������˳��Ϊfabcde;��ȷ��:fabcde����װ��A�������Ƿ�ֹ������ȫƿ;��ȷ��:��ֹ������ȫƿ��
�۹���μ�NaClO��Һ,����NaClO������ȫ��Ӧ,������NaClO��Һ�ܹ�������Ӧ������ˮ����,���½��Ͳ���;��ȷ��: ����μ�NaClO��Һ��������NaClO��Һ����ˮ���£����Ͳ�����
(2)�ܱ���60~70������·�����Ӧ,��˿��Բ���ˮԡ���ȵķ���;�����������������Һ��ַ�Ӧ������ɫ��NaIO��NaIO3,������ȫ����ʧ;���Ըò��跴Ӧ��ȫ�������������������Һ����ɫ;N2H4![]() H2O���л�ԭ��,�ܹ���I03-��ԭΪ������,��-2�۵ĵ�Ԫ�ر�����Ϊ����,���ӷ���ʽΪ: 2IO3��+ 3N2H4��H2O =3N2��+2I-+9H2O;��ȷ��:�����������Һ����ɫ; 2IO3��+ 3N2H4��H2O =3N2��+2I-+9H2O��
H2O���л�ԭ��,�ܹ���I03-��ԭΪ������,��-2�۵ĵ�Ԫ�ر�����Ϊ����,���ӷ���ʽΪ: 2IO3��+ 3N2H4��H2O =3N2��+2I-+9H2O;��ȷ��:�����������Һ����ɫ; 2IO3��+ 3N2H4��H2O =3N2��+2I-+9H2O��
��8.4gNaOH��25.4g���ʵⷴӦ���������ƹ���,�ⵥ�ʷ�Ӧ��ȫ,����������Ʒ�����ӦΪ3I2+6NaOH=5NaI+NaIO3+3H2O,�����ɵ�NaI������Ϊ25.4![]() 750/762=25g,���ɵ�NaIO��N2H4
750/762=25g,���ɵ�NaIO��N2H4![]() H2O��Ӧ���õ�NaI,��ӦΪ3N2H4
H2O��Ӧ���õ�NaI,��ӦΪ3N2H4![]() H20+2NaIO3=2NaI+3N2
H20+2NaIO3=2NaI+3N2![]() +9H2O,��6I2
+9H2O,��6I2![]() 2NaIO3
2NaIO3![]() 2NaI�ò����ɵ�NaI����Ϊ25.4
2NaI�ò����ɵ�NaI����Ϊ25.4![]() 300/1524=5g,�����������ɵ�NaIΪ25g+5g=30g,����,����ʵ��IJ���Ϊ24/30
300/1524=5g,�����������ɵ�NaIΪ25g+5g=30g,����,����ʵ��IJ���Ϊ24/30![]() 100%=80%;ˮ����ʵ������������ֵƫ��,ˮ���»�ԭ�Խ�ǿ,������ˮ�е��ܽ�����Ӧ;��ȷ��:80%;ˮ��������ˮ�е��ܽ�����Ӧ��
100%=80%;ˮ����ʵ������������ֵƫ��,ˮ���»�ԭ�Խ�ǿ,������ˮ�е��ܽ�����Ӧ;��ȷ��:80%;ˮ��������ˮ�е��ܽ�����Ӧ��
�߸÷���������,�����Ӿ���ǿ��ԭ��,�����������±����������ɵⵥ��,����ʹ������Һ����;��ȷ��:������1-�����Ի����б�O2������I2��ʹ���۱�����