��Ŀ����
����Ŀ����ϩ�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ����Ҫ��־��
I����8.96L (��״��)��ϩ������Ļ������ͨ��������������Ȼ�̼��Һ�У���ַ�Ӧ��������Ȼ�̼��Һ����������8.4g����ԭ������������ϩ����������ʵ���֮��Ϊ______________��
II����֪��ϩ�ܷ�������ת����
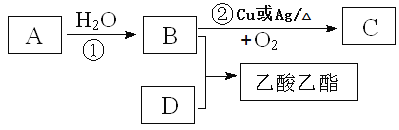
��д��B��D�������й����ŵ����ƣ�B____________________��D___________________��
��д����ط�Ӧ�Ļ�ѧ����ʽ��
��_________________________________ ��Ӧ���ͣ�________________
��__________________________________����Ӧ���ͣ�_________________
���𰸡�3��1 �ǻ� �Ȼ� CH2=CH2+ H2O��CH3CH2OH �ӳ� 2CH3CH2OH��O2![]() 2CH3CHO��2H2O ����
2CH3CHO��2H2O ����
��������
I����ϩ����˫����������ˮ�����ӳɷ�Ӧ����ϩ������Ļ������ͨ��������ˮ�У���ַ�Ӧ����ˮ������������8.4g����ϩ������Ϊ8.4g������n=![]() ������ϩ�����ʵ�����n=
������ϩ�����ʵ�����n=![]() ���������������ʵ���������������������ʵ������ɼ���������������ʵ���֮�ȣ�
���������������ʵ���������������������ʵ������ɼ���������������ʵ���֮�ȣ�
��������ͼ��֪����ϩ��ˮ��Ӧ�����Ҵ���B���Ҵ����Ҵ������������õ�����C����CΪ��ȩ���Ҵ���������Ũ���������·���������Ӧ��������������D������Դ˽����⡣
I��8.96L�����������ʵ���Ϊn=![]() 0.4mol����ϩ����˫����������ˮ�����ӳɷ�Ӧ����ϩ������Ļ������ͨ��������ˮ�У���ַ�Ӧ����ˮ������������8.4g����ϩ��������8.4g��������ϩ�����ʵ���Ϊn=
0.4mol����ϩ����˫����������ˮ�����ӳɷ�Ӧ����ϩ������Ļ������ͨ��������ˮ�У���ַ�Ӧ����ˮ������������8.4g����ϩ��������8.4g��������ϩ�����ʵ���Ϊn=![]() 0.3mol������������ʵ���Ϊ��0.4mol-0.3mol=0.1mol��ԭ������������ϩ����������ʵ���֮��Ϊ3��1��
0.3mol������������ʵ���Ϊ��0.4mol-0.3mol=0.1mol��ԭ������������ϩ����������ʵ���֮��Ϊ3��1��
II����B���Ҵ��������ŵ��������ǻ���D�����ᣬ�����ŵ��������Ȼ���
�Ʒ�Ӧ��Ϊ��ϩ�ڴ�����������ˮ�����ӳɷ�Ӧ�����Ҵ�����Ӧ����ʽ��CH2=CH2+ H2O��CH3CH2OH�� ��Ӧ����Ϊ�ӳɷ�Ӧ��
��Ӧ��Ϊ�Ҵ�������������ȩ����Ӧ����ʽΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O����Ӧ����Ϊ������Ӧ��
2CH3CHO+2H2O����Ӧ����Ϊ������Ӧ��

����Ŀ��2016��ȹ��ҿ�ѧ�����������ҹ�ŵ����������������ϣ��������ڿ�ű�������ط�����о���
���������ϣ��������۵�156~157�棬�����ڱ�ͪ���ȷº����ѣ���ˮ�������ܡ�
I.ʵ������������ȡ�����صĹ����������£�

��1���ڲ���IǰҪ��������з��飬��Ŀ����________________
��2������II��������_____________��
��3������III���е����ؽᾧ�����������Ϊ_____ ��_____��______�����ˡ�ϴ�ӡ�����
II����֪��������һ�����ĺ���������Ϊȷ�����Ļ�ѧʽ������������ʵ�飺

ʵ�鲽�裺������װ�ã����װ�õ������ԡ��ڳ���E��F��������ҩƷ����������ȡ14.10g�����ط���Ӳ���Թ�C�У���ȼC��D���ƾ��Ƽ��ȣ����ȼ�բ�ʵ���������ȴ�����£�������Ӧ��E��F��������ҩƷ��������
��4��װ��E��FӦ�ֱ�װ���ҩƷΪ_______________��___________________��
��5��ʵ���ã�
װ�� | ʵ��ǰ | ʵ��� |
E | 24.00g | 33.90g |
F | 100.00g | 133.00g |
ͨ��������������ص���Է�������Ϊ282������������ݣ��ó������صķ���ʽΪ______��
��6����ʹ����������������ϴ�ʵ������ĸĽ�������________________________��









