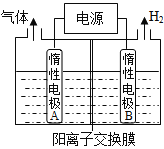
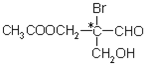
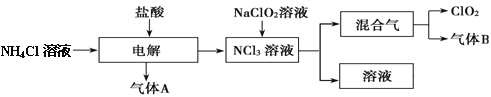
��Ŀ����
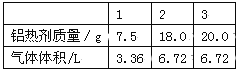
����Ŀ�����ۺ���������ĩ�Ļ�����Ƴɵ����ȼ������ں��Ӹֹ졣��ȡ���ݲ�ͬ�����ĸ����ȼ��ֱ��100mLͬŨ�ȵ�NaOH��Һ��Ӧ����ȡ���ȼ����������������������ϵ���±�������������ڱ�״���²ⶨ����
��1�������ȷ�Ӧ�Ļ�ѧ����ʽΪ ��
��2�������ȼ��м���NaOH��Һʱ������Ӧ�Ļ�ѧ����ʽΪ ��
��3����NaOH��Һ�����ʵ���Ũ�ȡ�
��4��������ȼ�����������������
���𰸡�
��1��2Al+Fe2O3![]() 2Fe+Al2O3��
2Fe+Al2O3��
��2��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��3��2mol/L��
(4) 36%��
��������
�����������1�����ȼ�����Ҫ�ɷ�Ϊ�������������ڸ��������·����û���Ӧ������������������Ӧ�Ļ�ѧ����ʽΪ2Al+Fe2O3![]() 2Fe+Al2O3����2������NaOH��Һ��Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2������3����������6.72Lʱ�������������ٱ仯��˵��NaOH��ȫ��Ӧ�����ݷ���ʽ������n(H2)=6.72L��22.4L/mol=0.3mol��
2Fe+Al2O3����2������NaOH��Һ��Ӧ����ƫ�����ƺ���������Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2������3����������6.72Lʱ�������������ٱ仯��˵��NaOH��ȫ��Ӧ�����ݷ���ʽ������n(H2)=6.72L��22.4L/mol=0.3mol��
2Al+2NaOH+2H2O=2NaAlO2+3H2��
2mol 2mol 3mol
n��NaOH�� 0.3mol
��n��NaOH��=0.2mol������c(NaOH)= 0.2mol ��0.1L=2mol/L����4���������ȼ�7.5gʱ��������ȫ��Ӧ����ʱn(H2)=3.36L��22.4L/mol=0.15mol�����ݷ���ʽ2Al+2NaOH+2H2O=2NaAlO2+3H2����Al�����������ʵ�����ϵ��֪n(Al)=2/3 n(H2)=0.1mol����m��Al��=0.1mol��27g/mol=2.7g�����������ȼ����������������� Al%=(2.7g��7.5g)��100%=36%��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�