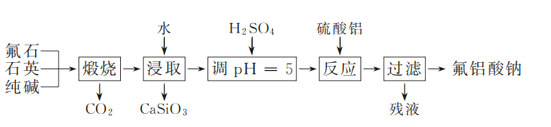
��Ŀ����
����Ŀ����ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ���̪��ָʾ�����ش��������⣺
��1���ñ�������ζ������NaOH��Һʱ���յ�������_______��
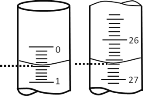
��2�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����ζ�����ʱ�Ķ���Ϊ___________ mL������������Һ�����Ϊ_______mL��
�ζ����� | ����NaOH��Һ�����/mL | 0.1000mol��L��1��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
��3��ijѧ������3��ʵ��ֱ��¼�й��������±���
�����ϱ����ݼ���ɵø�NaOH��Һ�����ʵ���Ũ��Ϊ___mol��L��1��������λ��Ч���֣���
��4�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���_____������ĸ����
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ƿ��ˮϴ�Ӻ��ô���Һ��ϴ
��5������к͵ζ�ԭ��Ҳ�������������͵ĵζ����磺һ�ֲⶨˮ����Br����Ũ�ȵ�ʵ�鲽�����£�
������ƿ�м��봦�����ˮ��25.00mL�����뼸��NH4Fe(SO4)2��Һ��
�ڼ���V1mL c1 mol/L AgNO3��Һ�������������ҡ�ȡ�
����c2mol/L KSCN����Һ���еζ������յ�ʱ���ı���ҺV2mL��
�����ˮ����Br�������ʵ���Ũ��Ϊ_______mol��L��1����֪��Ksp(AgBr��= 7.7��10��13��Ag++ SCN��=AgSCN(��ɫ)�� ��Ksp(AgSCN)= 1��10��12����
��ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L������Һ�����к��ȵIJⶨ��ʵ��װ����ͼ��ʾ��
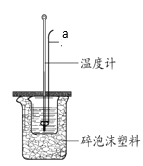
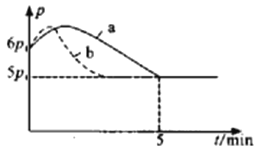
��6������a��������_______��
��7��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬�����ֹ�¶Ȳ��ƽ��ֵΪ4.0�档������Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���ͺ�������Һ�ı�����c��4.18 J/(g����)���������к�����H��______(ȡС�����һλ)��
��8������ʵ�����룭57.3 kJ/mol��ƫ�����ƫ���ԭ�������_____ (����ĸ)��
a��ʵ��װ�ñ��¡�����Ч����
b������Ͳ��ȡNaOH��Һ�����ʱ���ӿ̶��߶���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
���𰸡��������һ�α�Һ����Һ�Ӻ�ɫ�����ɫ��30s�ޱ仯 26.60 26.10 0.2088 B ![]() ���β�������� ��53.5kJ��mol-1 acd
���������� ��53.5kJ��mol-1 acd
��������
��1���ñ�������ζ������NaOH��Һʱ���յ������������ɫ��������ɫ��
��2�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����ζ�����ʱ�Ķ���Ϊ26.60mL������������Һ�����Ϊ26.10mL��
��3���ڶ���ʵ������ʧ�棬��һ������������������ƽ��ֵΪ26.10mL�������ϱ����ݼ���ɵø�NaOH��Һ�����ʵ���Ũ��Ϊ![]() ��
��
��4��A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������ᣬ����Ũ��ƫС���������ƫ���NaOH��Һ��Ũ����ֵƫ�ߣ�A�������⣻
B����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ�������ȡ�����������ֵƫС�����NaOH��Һ��Ũ����ֵƫ�ͣ�B�������⣻
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ����ȡ���������ƫ���NaOH��Һ��Ũ����ֵƫ�ߣ�C�������⣻
D����ƿ��ˮϴ�Ӻ��ô���Һ��ϴ�������������ƫ���NaOH��Һ��Ũ����ֵƫ��D�������⡣
��5����ΪKsp(AgBr)< Ksp(AgSCN)������KSCN������AgBr������Ӧ����ˮ����Br�������ʵ���Ũ��ΪAgNO3����ʼ���ʵ�����ʣ�����ʵ���֮�����ˮ���������
��6������a�������ǻ��β����������
��7��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬�����ֹ�¶Ȳ��ƽ��ֵΪ4.0�榤H��![]() ��
��
��8��a��ʵ��װ�ñ��¡�����Ч�����H��ֵƫС��
b������Ͳ��ȡNaOH��Һ�����ʱ���ӿ̶��߶�����NaOH��ȡ���ƫ���ͷŵ�����ƫ�࣬��H��ֵƫ��
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У��ڼ�������е���������ʧ����H��ֵƫС��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ����¶ȼ��ϸ��ŵ�NaOH�����ᷴӦ���ȣ������������ʼ�¶�ƫ�ߣ�����������ֵƫС����H��ֵƫС��
��1���ñ�������ζ������NaOH��Һʱ���յ������ǵ������һ�α�Һ����Һ�Ӻ�ɫ�����ɫ��30s�ޱ仯����Ϊ���������һ�α�Һ����Һ�Ӻ�ɫ�����ɫ��30s�ޱ仯��
��2�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����ζ�����ʱ�Ķ���Ϊ26.60mL������������Һ�����Ϊ26.10mL����Ϊ��26.60��26.10��
��3���ڶ���ʵ������ʧ�棬��һ������������������ƽ��ֵΪ26.10mL�������ϱ����ݼ���ɵø�NaOH��Һ�����ʵ���Ũ��Ϊ![]() =0.2088����Ϊ��0.2088��
=0.2088������0.2088��
��4��A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������ᣬ����Ũ��ƫС���������ƫ���NaOH��Һ��Ũ����ֵƫ�ߣ�A�������⣻
B����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ�������ȡ�����������ֵƫС�����NaOH��Һ��Ũ����ֵƫ�ͣ�B�������⣻
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ����ȡ���������ƫ���NaOH��Һ��Ũ����ֵƫ�ߣ�C�������⣻
D����ƿ��ˮϴ�Ӻ��ô���Һ��ϴ�������������ƫ���NaOH��Һ��Ũ����ֵƫ��D�������⡣��Ϊ��B��
��5����ΪKsp(AgBr)< Ksp(AgSCN)������KSCN������AgBr������Ӧ����ˮ����Br�������ʵ���Ũ��Ϊ![]() mol��L��1����Ϊ��
mol��L��1������![]() ��
��
��6������a�������ǻ��β������������Ϊ�����β����������
��7����H��![]() =��53.5kJ/mol��������53.5kJ/mol��
=��53.5kJ/mol��������53.5kJ/mol��
��8��53.5<57.3������H�IJⶨֵƫ�͡�
a��ʵ��װ�ñ��¡�����Ч�����H��ֵƫС��
b������Ͳ��ȡNaOH��Һ�����ʱ���ӿ̶��߶�����NaOH��ȡ���ƫ���ͷŵ�����ƫ�࣬��H��ֵƫ��
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У��ڼ�������е���������ʧ����H��ֵƫС��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ����¶ȼ��ϸ��ŵ�NaOH�����ᷴӦ���ȣ������������ʼ�¶�ƫ�ߣ�����������ֵƫС����H��ֵƫС��
��acd�������⡣��Ϊ��acd��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�