��Ŀ����
����Ŀ��������̼��DZ�ڵ�̼��Դ����������Ȼ�Ķ�����̼���أ����Ǹ���¯����β���������������з�����պ���Ũ���������ã������ش�
(1)�ڿռ�վ�г�����CO2(g)+2H2(g)![]() C(s)+2H2O(g)���ٵ��ˮʵ��O2��ѭ�����ã�350��ʱ�������Ϊ2L�ĺ����ܱ�������ͨ��8molH2��4molCO2�������Ϸ�Ӧ��
C(s)+2H2O(g)���ٵ��ˮʵ��O2��ѭ�����ã�350��ʱ�������Ϊ2L�ĺ����ܱ�������ͨ��8molH2��4molCO2�������Ϸ�Ӧ��
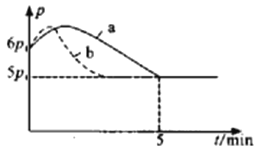
������Ӧ��ʼ��ƽ��ʱ�¶���ͬ(��Ϊ350��)����÷�Ӧ������ѹǿ(p)��ʱ��(t)�ı仯��ͼ��a��ʾ����������Ӧ�ġ�H___________0(�>����<��)������������ͬʱ�������ı�ijһ�����������ѹǿ(p)��ʱ��(t)�ı仯��ͼ������b��ʾ����ı��������___________��
��ͼ�Ƿ�Ӧƽ�ⳣ���Ķ������¶ȵı仯��ϵͼ��m��ֵΪ___________��

(2)CO2�� Cu-ZnO���£�ͬʱ�������·�ӦI��II���ǽ������ЧӦ����Դ��ȱ����Ҫ�ֶΡ�
��.CO2(g)+3H2(g)![]() CH3OH (g)+H2O(g) ��H1<0
CH3OH (g)+H2O(g) ��H1<0
��.CO2(g)+H2(g)![]() CO(g)+ H2O(g) ��H2>0
CO(g)+ H2O(g) ��H2>0
�����¶�Tʱ�����ݻ�������ܱ������У�����һ������CO2��H2����ʼ����ƽ��ʱ�������ڸ��������ʵ�������ѹǿ���±���

����ӦI��II����ƽ��ʱ��p0=1.4p�������n=__________����Ӧ1��ƽ�ⳣ��Kp=____ (kPa)��2��(�ú�p��ʽ�ӱ�ʾ)
(3)Al-CO2�����һ���Ե���������[Al2(CO3)3]Ϊ����ʣ�����ȫ��̼����Pd�����������Ϊ���������Ŀɳ���ء�������ӦΪ��3CO2+4e��=2CO32��+C��������Al�ķ�Ӧ������___________��(�����������)���õ�س��ʱ��Ӧ�Ļ�ѧ����ʽΪ___________��
���𰸡�< ������� 0 0.2 ![]() �� 2Al2��CO3��3+3C
�� 2Al2��CO3��3+3C![]() 4Al+9CO2
4Al+9CO2
��������
(1)�ٸ���PV=nRT�жϣ��۲�����յ�ı仯ȷ���ı��������
��������ʽ���м��㣻
(2)���ݱ����ҵ�������ƽ��ʱ�����ʵ������ڼ���ƽ�ⳣ����
(3)��������ʧ�������������û������ʱ�����������Ӹ���
(1�ٸ���PV=nRT���˷�ӦVһ��������Ӧn�ڼ�С��ͼ����ʾѹǿ������ȷ���¶������ߣ���������ӦΪ���ȷ�Ӧ����H<0������ͼ���������b�������a�߲�ͬ��ֻ�����ʼӿ죬�ﵽƽ��ʱ�����̣�ȷ���ı�������Ǵ�����
�𰸣�< �������
��������ʽ��
CO2(g)+ 2H2(g)![]() C(s)+2H2O(g)
C(s)+2H2O(g)
c������ 2 4 0
��c x 2x 2x
c��ƽ��2-x 4-2x 2x
(2-x+4-2x+2x)/(2+4)=5/6
x=1
K=![]() =1,lgK=lg1=0
=1,lgK=lg1=0
�𰸣�0
(2)���ݱ����г����������ʵ����仯��
�� CO2(g)+3H2(g)![]() CH3OH (g)+H2O(g) ��H1<0
CH3OH (g)+H2O(g) ��H1<0
��n n 3n n n
��.CO2(g)+H2(g)![]() CO(g)+ H2O(g) ��H2>0
CO(g)+ H2O(g) ��H2>0
��n 0.3-n 0.3-n 0.3-n 0.3-n
ƽ��ʱ�����ʵ���Ϊ��CO2(g)��0.5-n-0.3+n=0.2mol H2(g):0.9-3n-0.3+n=0.6-2n
CH3OH (g):n H2O(g):0.3 CO(g):0.3-n
����T��Vһ��ʱ��ѹǿ�ȵ������ʵ���֮�ȼ���n
![]() =
=![]() =1.4,����ó�n=0.2��
=1.4,����ó�n=0.2��
ƽ������ʵ�������0.2+0.6-2��0.2+0.2+0.3+0.3-0.2=1.0mol
ƽ�������ʷ�ѹ�� CO2(g):��0.2/1��P=0.2P H2(g) :(0.2/1)P=0.2P CH3OH (g):(0.2/1)P=0.2P H2O(g):(0.3/1)P=0.3P
Kp=![]() =
=![]()
�𰸣�0.2 ![]()
(3)��Ϊ�ŵ�ʱAl-3e-=Al3+������ʱӦ����Al3++3e-=Al���õ���Ϊ������Ӧ�����ʱ�ܷ�Ӧ����ʽΪ�ŵ�ʱ���淴Ӧ��
�𰸣��� 2Al2��CO3��3+3C![]() 4Al+9CO2
4Al+9CO2

 �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
