题目内容
【题目】(Ⅰ)A、B、C为三种强电解质,它们在水中电离出的离子如下表所示:
阳离子 | Na+、K+、Cu2+ |
阴离子 | SO42-、OH- |
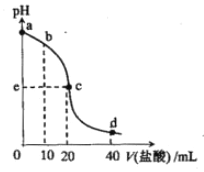
如图1所示装置中,甲、乙、丙三个烧杯中依次盛放足量的A溶液、足量的B溶液、足量的C溶液,电极均为石墨电极。接通电源,经过一段时间后,测得乙中c电极质量增加了32g。常温下各烧杯中溶液的pH与电解时间t的关系如图2所示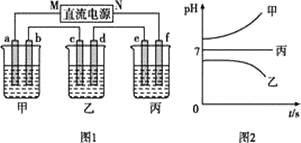
请回答下列问题:
(1)N为电源的(填“正”或“负”)极,电极b上发生的电极反应为。
(2)乙烧杯中的总反应为。
(3)计算电极e上生成的气体在标准状况下的体积为。
(4)丙烧杯要恢复到原来的状况,需要加入的物质和质量是。
(5)(Ⅱ)粗铜中一般含有锌、铁、银、金等杂质。在下图所示装置中,甲池的总反应方程式为:2CH3OH+3O2+4KOH═2K2CO3+6H2O 接通电路一段时间后,精Cu电极质量增加了3.2g。在此过程中,甲池负极反应式 , 乙池硫酸铜溶液的浓度(填“变大”,“ 不变”,“变小”)。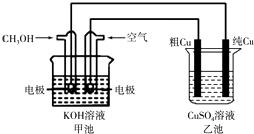
【答案】
(1)正;4OH--4e-=2H2O+O2↑
(2)2CuSO4+2H2O ![]() 2Cu+O2↑+2H2SO4
2Cu+O2↑+2H2SO4
(3)11.2 L
(4)水;9g
(5)CH2OH-6e-+8OH-=CO3-+6H2O;变小
【解析】接通电源,经过一段时间后,测得乙中c电极质量增加了16g,则乙应为CuSO4溶液,c为阴极,d为阳极,则a为阴极,b为阳极,e为阴极,f为阳极,M为负极,N为正极,常温下各烧杯中溶液的pH与电解时间t的关系图如图2,甲的pH不断增大,应为氢氧化钠或氢氧化钾溶液,丙的pH不变,应为硫酸钠或硫酸钾溶液,
(1)由以上分析可知N为正极,甲为电解氢氧化钠或氢氧化钾溶液,b为阳极,发生氧化反应,电极方程式为4OH--4e-=2H2O+O2↑;
(2)乙应为CuSO4溶液,电解时,阴极析出铜,阳极生成氧气,电解方程式为2CuSO4+2H2O ![]() 2Cu+O2↑+2H2SO4;
2Cu+O2↑+2H2SO4;
(3)n(Cu)= ![]() =0.5 mol,转移电子1 mol,则电极e发生:2H++2e-=H2↑,则生成n(H2)=0.5 mol,v(H2)=0.5 mol×22.4L/mol=11.2 L;
=0.5 mol,转移电子1 mol,则电极e发生:2H++2e-=H2↑,则生成n(H2)=0.5 mol,v(H2)=0.5 mol×22.4L/mol=11.2 L;
(4)丙为硫酸钠或硫酸钾溶液,实质上为电解水,转移电子1 mol时,2H2O ![]() 2H2↑+O2↑,消耗水的物质的量为0.5 mol,质量为0.5 mol×18g/mol=9 g.(Ⅱ)
2H2↑+O2↑,消耗水的物质的量为0.5 mol,质量为0.5 mol×18g/mol=9 g.(Ⅱ)
(5)甲池中,通入甲醇的电极是负极,碱性条件下,电极反应式中不能产生氢离子,电极反应式为:CH3OH-6e-+8OH-═CO32-+6H2O,乙池中,阳极上不仅溶解铜还溶解锌、铁、银等金属,阴极上只有铜析出,所以阳极上溶解的铜小于阴极上析出的铜,则溶液中硫酸铜溶液浓度减小。接通电源,经过一段时间后,测得乙中c电极质量增加了16g,则乙应为CuSO4溶液,c为阴极,d为阳极,则a为阴极,b为阳极,e为阴极,f为阳极,M为负极,N为正极,常温下各烧杯中溶液的pH与电解时间t的关系图如图2,甲的pH不断增大,应为氢氧化钠或氢氧化钾溶液,丙的pH不变,应为硫酸钠或硫酸钾溶液,据此解答即可。

 名师指导期末冲刺卷系列答案
名师指导期末冲刺卷系列答案