题目内容
【题目】(Ⅰ)50mL0.5mol·L-1的盐酸与50mL0.55mol·L-1的NaOH溶液在下图所示的位置中进行中和反应。通过测定反应过程中放出的热量可计算中和热。回答下列问题:
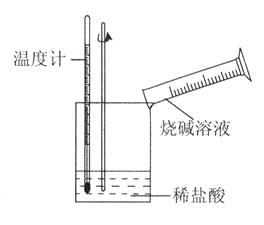
(1)从实验装置上看,可知下图装置有三处不妥之处,请指出。
(2)在测定中和热的实验中,计算反应热时需要的数据有_________
①酸的浓度和体积 ②碱的浓度和体积 ③比热容 ④反应前后溶液的温度差 ⑤操作所需时间
A.①②③⑤
B.①②③④
C.②③④⑤
D.①③④⑤
(3)实验中改用60mL0.50mol·L-1的盐酸跟50mL0.55mol·L-1的NaOH溶液进行反应,与上述实验相比,所求中和热(填“相等”或“不相等”),所放出的热量(填“相等”或“不相等”)。
(4)用相同浓度和体积的氨水代替NaOH溶液进行上述实验,测得的中和热数值会(填“偏大”、“偏小”或“无影响”).
(5)(Ⅱ)化学反应可视为旧键断裂和新键形成的过程,化学键的键能是形成(或拆开)1mol化学键时释放(或吸收)的能量,已知白磷和P4O6 的分子结构如下图所示,现提供以下化学键的键能: ![]() :
: ![]() kJ·mol-1 ,
kJ·mol-1 , ![]() :
: ![]() kJ·mol-1 ,
kJ·mol-1 , ![]() :
: ![]() kJ·mol-1 , 则反应P4(白磷) +3O2=P4O6的热化学反应方程式为。
kJ·mol-1 , 则反应P4(白磷) +3O2=P4O6的热化学反应方程式为。
(6)肼(N2H4)可作为火箭发动机的燃料,与氧化剂N2O4反应生成N2和水蒸气。
已知:①N2(g)+2O2(g)═N2O4(l) △H1═-19.5kJmol-1
②N2H4(l)+O2(g)═N2(g)+2H2O(g) △H2═-534.2kJmol-1
写出肼和N2O4反应的热化学方程式 。
(7)化学反应N2+3H2 ![]() 2NH3的能量变化如图所示,该反应生成NH3(I)的热化学方程式是。
2NH3的能量变化如图所示,该反应生成NH3(I)的热化学方程式是。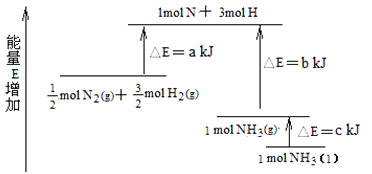
【答案】
(1)无环形玻璃搅拌棒;在大小烧杯间没有填满碎泡沫(或纸条);小烧杯口与大烧杯口不相等
(2)B
(3)不等;相等
(4)偏小
(5)P4(白磷 s)+N2O4(I)=3N2(g)+2H2O(g)
(6)△H=-1638kJ·mol-12N2H4(I)+N2O4(I)=3N2(g)+4H2O(g) △H=-1048.9kJ/mol
(7)N2(g)+3H2(g) ![]() 2NH3(I) △H=2(a-b-c)kJ·mol-1
2NH3(I) △H=2(a-b-c)kJ·mol-1
【解析】(1)为了测得温度的最高值,应在最短的时间内让盐酸和氢氧化钠充分反应,故缺少环形玻璃搅拌棒;为了测得温度的最高值,应加强保温、隔热和防止热量散失措施,应在在大小烧杯间填满碎泡沫(或纸条),并使小烧杯口与大烧杯口相平;
(2)在测定中和热的实验中,计算反应热时需要的数据有酸的浓度和体积、碱的浓度和体积、比热容、反应前后溶液的温度差,与实验操作所需时间无关,故选项B符合题意;
(3)反应放出的热量和所用酸以及碱的量的多少有关,并若用60mL0.50molL-1盐酸与50mL0.55molL-1NaOH溶液进行反应,与上述实验相比,生成水的量增多,所放出的热量偏高,但是中和热的均是强酸和强碱反应生成1mol水时放出的热,与酸碱的用量无关,中和热数值相等;
(4)氨水为弱碱,电离过程为吸热过程,所以用氨水代替稀氢氧化钠溶液反应,反应放出的热量小于57.3kJ;
(5)白磷燃烧放出的热量=生成物的键能-反应物的键能=12×360 kJ-(6×198)kJ-(3×498)kJ=1638 kJ,反应的焓变是-1638 KJ/mol;反应P4(白磷)燃烧生成P4O6的热化学方程式为:P4(s)+3O2(g)=P4O6(g)△H=-1638 KJ/mol;
(6)N2(g)+2O2(g)=N2O4(l)△H1=-19.5kJmol-1②N2H4(l)+O2 (g)=N2(g)+2H2O(g)△H2=-534.2kJmol-1 根据盖斯定律写出肼和N2O4反应的热化学方程:②×2-①得到:2N2H4(l)++N2O4(l)=3N2(g)+4H2O(g)△H=-1048.9 kJ/mol;
(7)由图可知,生成1mol NH3(g)放出的热量为b-akJ,由气体变为液体,放出热量为ckJ,则N2(g)+3H2(g)=2NH3(1)△H=-2(b-a)kJ/mol+2(-c)kJ/mol=2(a-b-c)kJmol-1。

【题目】(Ⅰ)A、B、C为三种强电解质,它们在水中电离出的离子如下表所示:
阳离子 | Na+、K+、Cu2+ |
阴离子 | SO42-、OH- |
如图1所示装置中,甲、乙、丙三个烧杯中依次盛放足量的A溶液、足量的B溶液、足量的C溶液,电极均为石墨电极。接通电源,经过一段时间后,测得乙中c电极质量增加了32g。常温下各烧杯中溶液的pH与电解时间t的关系如图2所示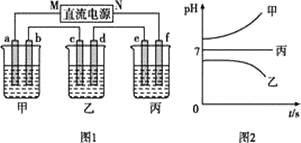
请回答下列问题:
(1)N为电源的(填“正”或“负”)极,电极b上发生的电极反应为。
(2)乙烧杯中的总反应为。
(3)计算电极e上生成的气体在标准状况下的体积为。
(4)丙烧杯要恢复到原来的状况,需要加入的物质和质量是。
(5)(Ⅱ)粗铜中一般含有锌、铁、银、金等杂质。在下图所示装置中,甲池的总反应方程式为:2CH3OH+3O2+4KOH═2K2CO3+6H2O 接通电路一段时间后,精Cu电极质量增加了3.2g。在此过程中,甲池负极反应式 , 乙池硫酸铜溶液的浓度(填“变大”,“ 不变”,“变小”)。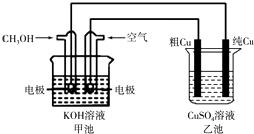
【题目】丙烯酸甲脂是—种重要的工业原料,某实验小组制取丙烯酸甲脂的装置如图所示: 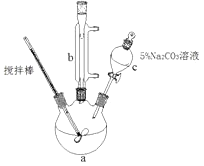
CH2=CHCOOH+HOCH3 ![]() CH2=CHCOOCH3+H2O
CH2=CHCOOCH3+H2O
①取10.0g丙烯酸和6.0g甲醇放置于三颈烧瓶中,连接好冷凝管,用搅拌棒搅拌,水浴加热。
②充分反应后,冷却,向混合液中加入5%Na2CO3溶液洗至中性。
③分液,取上层油状液体,再用无水Na2SO4干燥后蒸馏,收集70-90℃馏分。
可能用到的信息:
沸点 | 溶解性 | ||
丙烯酸 | 141℃ | 与水互溶,易溶于有机溶剂 | 有毒 |
甲醇 | 65℃ | 与水互溶,易溶于有机溶剂 | 易挥发,有毒 |
丙烯酸甲酯 | 80.5℃ | 难溶于水,易溶于有机溶剂 | 易挥发 |
回答下列问题:
(1)仪器b的名称是。
(2)混合液用5%Na2CO3溶液洗涤的目的是。
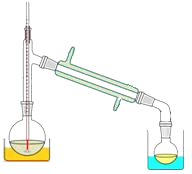
(3)关于产品的蒸馏操作(夹持装置未画出),下图中有2处错误,请分别写出、。![]()
![]()
为检验产率,设计如下实验:
①将油状物质提纯后平均分成5份,取出1份置于锥形瓶中,加入2.5mol/L的KOH溶液10.00mL,加热使之完全水解。
②用酚酞做指示剂,向冷却后的溶液中滴加0.5mol/L的HCl溶液,中和过量的KOH,滴到终点时共消耗盐酸18.00mL。
(4)计算本次酯化反应丙烯酸的转化率。
(5)请列举2条本实验中需要采取的安全防护措施、。