��Ŀ����
����Ŀ���ϳ���(CO+H2) �㷺���ںϳ��л����ҵ�ϳ�������Ȼ����ˮ������Ӧ�ȷ�������ȡ�ϳ�����
(1)��֪����£�5.6LCH4��ˮ������ȫ��Ӧʱ����51.5kJ����������д���÷�Ӧ���Ȼ�ѧ����ʽ________________��
(2)��150��ʱ2L ���ܱ������У���2molCH4��2mol H2O(g)��ϣ�����15min�ﵽƽ�⣬��ʱCH4��ת����Ϊ60%���ش��������⣺
�ٴӷ�Ӧ��ʼ��ƽ�⣬�������ı仯������ʾ�÷�Ӧ����v(H2)=________��
���ڸ��¶��£�����÷�Ӧ��ƽ�ⳣ��K=________��
������ѡ�����ܱ�ʾ�÷�Ӧ�Ѵﵽƽ��״̬����________��
A��V(H2)��=3v (CO)�� B���ܱ������л��������ܶȲ���
C���ܱ���������ѹǿ���� D��C(CH4)=C(CO)
(3)�ϳ����е�����Ҳ���ںϳɰ�����N2+3H2![]() 2NH3�������¶Ⱥ�������䣬�ڼס��ҡ������������н���ƽ��������Ϣ���±���������˵����ȷ����_______________��
2NH3�������¶Ⱥ�������䣬�ڼס��ҡ������������н���ƽ��������Ϣ���±���������˵����ȷ����_______________��
���� | ��� | ��ʼ���� | ƽ��ʱNH3�����ʵ��� | ƽ��ʱN2 ��������� | ��Ӧ��ʼʱ������ | ƽ��ʱ������ѹǿ |
�� | 1L | 1molN2+3molH2 | 1.6mol | ���� | v�� | P�� |
�� | 1L | 2molN2+6molH2 | n1 mol | ���� | v�� | P�� |
�� | 2L | 2molN2+6molH2 | n2 mol | ���� | v�� | P�� |
A��n1=n2=3.2 B������=����>���� C��v�� >v>v�� D��P��>P��=P��
(4)�ϳ���������ȡ���ѣ���ɫ��Դ��������-����ȼ�ϵ�ء�����ԭ������ͼ��ʾ
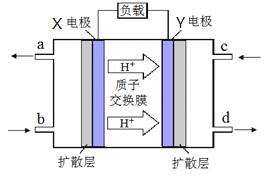
�� �缫Y �Ϸ����ķ�ӦʽΪ__________ ��
�ڵ���ڷŵ�����У��缫X��Χ��Һ��pH_______(�����С�����䡱)��
���𰸡� CH4(g)+H2O(g)=CO(g)+3H2(g) ��H= +206kJ/mol 0.12mol��L-1��min-1 21.87 AC BD O2+4e-+4H+=2H2O ��С
��������(1). �����5.6LCH4Ϊ0.25mol����ʽΪ: CH4(g)+H2O(g)=CO(g)+3H2(g)�����ȷ�Ӧ����HΪ������H=+51.5��4=+206kJ/mol��
(2). �ټ���ת��1.2mol����������3.6mol���������ı仯��Ϊ3.6mol/2L/15min=0.12 mol��L-1��min-1����![]() ����A. �ӷ�Ӧ��ʼ��ƽ�������ı仯��ΪCO(g)��3����A��ȷ��B. ��Ӧ�����غ㣬���۷�Ӧ��Σ����������䣬����������䣬���ܶȲ��䣻C. �����������������ͬ����������ѹǿ���䣬������������仯������Ӧ�ﵽƽ�⣬C��ȷ��D. ��Ӧƽ��ʱCH4��ת����Ϊ��һ��Ϊ50%����C(CH4)= C(CO)���ܱ�֤��Ӧƽ�⣬D��������ѡ��AC��
����A. �ӷ�Ӧ��ʼ��ƽ�������ı仯��ΪCO(g)��3����A��ȷ��B. ��Ӧ�����غ㣬���۷�Ӧ��Σ����������䣬����������䣬���ܶȲ��䣻C. �����������������ͬ����������ѹǿ���䣬������������仯������Ӧ�ﵽƽ�⣬C��ȷ��D. ��Ӧƽ��ʱCH4��ת����Ϊ��һ��Ϊ50%����C(CH4)= C(CO)���ܱ�֤��Ӧƽ�⣬D��������ѡ��AC��
(3).�������е���ʼѹǿ���ڱ������е���ʼѹǿ����n1n2����ȣ�A����B. �����ͱ�����ʼѹǿ�����С������������������Ӧ�̶Ȧ���>����=����������N2 �������������=����>������B��ȷ��C. ͬB��v�� >v��=v����C����D. ���ڷ���������������ʵ����������仯������ƽ��ʱ����ѹǿ������ʼѹǿ����P��>P��=P����D��ȷ������ѡ��BD��
(4). ����Ϊ��������Y���ƶ�����Y���õ�����Ϊ��������ȼ�ϵ��������Ϊ��Ӧ��䷴ӦΪ��O2+4e-+4H+=2H2O���ڵ���ڷŵ�����У�X�������������ӣ�����pH��С��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�