��Ŀ����
����Ŀ����ҵ���úϳ���(CO��H2)��ȡ�Ҵ��ķ�ӦΪ2CO+4H2![]() CH3CH2OH+H2O���о����֣�ʹ��TiO2��Ϊ���帺������������нϸߵ��Ҵ��������ش��������⣺
CH3CH2OH+H2O���о����֣�ʹ��TiO2��Ϊ���帺������������нϸߵ��Ҵ��������ش��������⣺
��1��Ti��̬ԭ�Ӻ�������Ų�ʽΪ_________����Oͬһ������Ԫ�صĵ�һ�����ܱ�O�����______����Ԫ�ط��ţ�����Oͬһ�����һ�̬ԭ�Ӻ���δ�ɶԵ�������O�����____����Ԫ�ط��ţ���
��2��H2O������Oԭ�ӵļ۲���Ӷ�����________��CH3CH2OH�������Ǽ�(-CH2-)�ϵ�Cԭ�ӵ��ӻ���ʽΪ_______��
��3�����úϳ�����ȡ�Ҵ���Ӧ���漰��4�������У��е�ӵ͵��ߵ�˳��Ϊ_________��ԭ����__________��
��4����ҵ����CO��O2��NH3Ϊԭ�ϣ��ɺϳɵ�������[CO(NH2)2]��CO(NH2)2�����к��е���������������Ŀ֮��Ϊ_________��
��5��CԪ����NԪ���γɵ�ij�־���ľ�����ͼ��ʾ��8��̼ԭ��λ��������Ķ��㣬4��̼ԭ��λ������������ģ�4����ԭ�����������ڣ����þ���Ӳ�ȳ������ʯ����Ϊ����һָ�ij�Ӳ�²��ϡ�
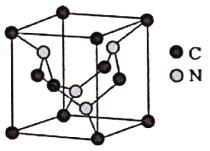
�ٸþ���Ӳ�ȳ������ʯ��ԭ����____________��
����֪�þ������ܶ�Ϊd g/cm3��Nԭ�ӵİ뾶Ϊr1cm��Cԭ�ӵİ뾶Ϊr2cm����NAΪ�����ӵ���������þ����Ŀռ�������Ϊ________(�ú�d��r1��r2��NA�Ĵ���ʽ��ʾ����
���𰸡� ls22s22p63s23p63d24s2��[Ar]3d24s2 N��F��Ne N 4 sp3 H2 <CO<CH3CH2OH< H2O H2Ϊ�Ǽ��Է��ӣ�COΪ���Է�����CO����Է���������H2�Ĵ����CO�ķ��»�����H2�Ĵ�H2O��CH3CH2OH��Ϊ���Ӽ��������ļ��Է��ӣ���ˮ�����γɵ������Ŀ���Ҵ����Ӷ� 7��1 �þ����е�C-N���ļ����Ƚ��ʯ�е�C-C���ļ����̣����ܴ�Ӳ�ȽϽ��ʯ�� ![]()
����������1��Ti��̬ԭ�Ӻ�������Ų�ʽΪls22s22p63s23p63d24s2��[Ar]3d24s2����Oͬһ������Ԫ�صĵ�һ�����ܱ�O�����N��F��Ne����Oͬһ�����һ�̬ԭ�Ӻ���δ�ɶԵ�������O��2��δ�ɶԵ��ӣ����ֻ��N��3������
��2��H2O������Oԭ�ӵļ۲���Ӷ�����![]() 4��CH3CH2OH�������Ǽ�(-CH2-)�ϵ�Cԭ���DZ��͵�Cԭ�ӣ��������ӻ���ʽΪsp3��
4��CH3CH2OH�������Ǽ�(-CH2-)�ϵ�Cԭ���DZ��͵�Cԭ�ӣ��������ӻ���ʽΪsp3��
��3�����úϳ�����ȡ�Ҵ���Ӧ���漰��4�������У��е�ӵ͵��ߵ�˳��ΪH2 <CO<CH3CH2OH< H2O��ԭ������H2Ϊ�Ǽ��Է��ӣ�COΪ���Է�����CO����Է���������H2�Ĵ����CO�ķ��»�����H2�Ĵ�H2O��CH3CH2OH��Ϊ���Ӽ��������ļ��Է��ӣ���ˮ��������2���ǻ���ԭ�Ӷ��Ҵ�ֻ��1���ǻ���ԭ�ӣ�����ˮ����֮���γɵ������Ŀ���Ҵ����Ӷࡣ
��4��CO(NH2)2�����к��е�7��������1����������������������Ŀ֮��Ϊ7��1��
��5���ɸþ���ľ����ṹ��֪���þ������е�Cԭ����Ϊ (8![]() )=3����Nԭ����Ϊ4�����û�����Ļ�ѧʽΪC3N4��
)=3����Nԭ����Ϊ4�����û�����Ļ�ѧʽΪC3N4��
�ٸþ���Ӳ�ȳ������ʯ��ԭ�������þ�����ԭ�Ӿ��壬Nԭ�ӵİ뾶��Cԭ��С�����Ըþ����е�C-N���ļ����Ƚ��ʯ�е�C-C���ļ����̣����ܴ�Ӳ�ȽϽ��ʯ��
����֪�þ������ܶ�Ϊd g/cm3��Nԭ�ӵİ뾶Ϊr1cm��Cԭ�ӵİ뾶Ϊr2cm����NAΪ�����ӵ���������NA��������1mol C3N4��������Ϊ92g�������Ϊ![]() ��4mol Nԭ�ӵ����Ϊ
��4mol Nԭ�ӵ����Ϊ![]() ��3mol Cԭ�ӵ����Ϊ
��3mol Cԭ�ӵ����Ϊ![]() �����Ըþ����Ŀռ�������Ϊ
�����Ըþ����Ŀռ�������Ϊ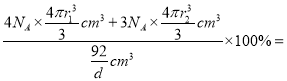
![]() ��
��

����Ŀ���ϳ���(CO+H2) �㷺���ںϳ��л����ҵ�ϳ�������Ȼ����ˮ������Ӧ�ȷ�������ȡ�ϳ�����
(1)��֪����£�5.6LCH4��ˮ������ȫ��Ӧʱ����51.5kJ����������д���÷�Ӧ���Ȼ�ѧ����ʽ________________��
(2)��150��ʱ2L ���ܱ������У���2molCH4��2mol H2O(g)��ϣ�����15min�ﵽƽ�⣬��ʱCH4��ת����Ϊ60%���ش��������⣺
�ٴӷ�Ӧ��ʼ��ƽ�⣬�������ı仯������ʾ�÷�Ӧ����v(H2)=________��
���ڸ��¶��£�����÷�Ӧ��ƽ�ⳣ��K=________��
������ѡ�����ܱ�ʾ�÷�Ӧ�Ѵﵽƽ��״̬����________��
A��V(H2)��=3v (CO)�� B���ܱ������л��������ܶȲ���
C���ܱ���������ѹǿ���� D��C(CH4)=C(CO)
(3)�ϳ����е�����Ҳ���ںϳɰ�����N2+3H2![]() 2NH3�������¶Ⱥ�������䣬�ڼס��ҡ������������н���ƽ��������Ϣ���±���������˵����ȷ����_______________��
2NH3�������¶Ⱥ�������䣬�ڼס��ҡ������������н���ƽ��������Ϣ���±���������˵����ȷ����_______________��
���� | ��� | ��ʼ���� | ƽ��ʱNH3�����ʵ��� | ƽ��ʱN2 ��������� | ��Ӧ��ʼʱ������ | ƽ��ʱ������ѹǿ |
�� | 1L | 1molN2+3molH2 | 1.6mol | ���� | v�� | P�� |
�� | 1L | 2molN2+6molH2 | n1 mol | ���� | v�� | P�� |
�� | 2L | 2molN2+6molH2 | n2 mol | ���� | v�� | P�� |
A��n1=n2=3.2 B������=����>���� C��v�� >v>v�� D��P��>P��=P��
(4)�ϳ���������ȡ���ѣ���ɫ��Դ��������-����ȼ�ϵ�ء�����ԭ������ͼ��ʾ
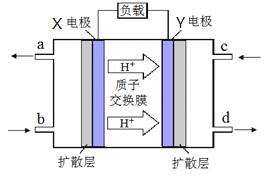
�� �缫Y �Ϸ����ķ�ӦʽΪ__________ ��
�ڵ���ڷŵ�����У��缫X��Χ��Һ��pH_______(�����С�����䡱)��
����Ŀ�������ֶ�����Ԫ�أ����ǵĽṹ�����ʵ���Ϣ���������
Ԫ�� | �ṹ�����ʵ���Ϣ |
X | �����л���ĺ���Ԫ�أ���Ԫ�ص�һ�����������̬�⻯�ﶼ�ǵ��͵��������� |
Y | ��������(��ϡ��������)ԭ�Ӱ뾶����Ԫ�أ��õ�������ˮ���ҷ�Ӧ |
Z | ��Yͬ���ڣ�������������ˮ��������� |
M | ��ˮ�г��⡢��Ԫ���⺬������Ԫ�أ��䵥�ʻ���Ҳ������ˮ���������г��õ���������ɱ���� |
����ݱ�����Ϣ��д��
(1)XԪ�������ڱ��е�λ��________������Է���������С����̬�⻯�ﳣ����______��
(2)��ҵ����ȡY���ʳ��õķ�����(�û�ѧ����ʽ��ʾ)_________��
(3)Y���Ӱ뾶��Z���ӵİ뾶________(���С��)��
(4)Z�ĵ��ʺ���������Ӧ������Ұ�⺸�Ӹֹ죬�÷�Ӧ����________(����ȡ����ȡ�)��Ӧ��д����Ӧ�Ļ�ѧ����ʽΪ______________��
(5)�ٳ�ʵ��˵��M�ķǽ����Ա�Xǿ��_____________��