��Ŀ����
����Ŀ����Ԫ���ܹ��γɶ��ֻ������ش��������⣺
��1������(N2H4)������ΪҺ̬���ڿ�����Ѹ����ȫȼ������N2��ͬʱ�������ȣ���������������ɴ��������ȼ�ϡ�
��֪��H2(g)+1/2O2(g) = H2O(l) ��H1= ��285.8kJ/mol
N2(g)+2H2(g) = N2H4(l) ��H2= + 50.6kJ/mol
��N2H4(l)�ڿ���ȼ������Һ̬ˮ���Ȼ�ѧ����ʽΪ_______________________��
��2�����ð�������������(HCN)�ķ�ӦCH4(g)+NH3(g)=HCN(g)+3H2(g)��H>0
��һ���¶��£���2L���������г���1molCH4(g)��2molNH3(g)����������Ӧ��4min�ﵽƽ��ʱ�����CH4ƽ��ת����Ϊ66.67%��0~4min�ڣ���H2��ʾ�ĸ÷�Ӧ����v(H2)=___________�������¶Ⱥ��ݻ����䣬����ƽ���������г���2molNH3��2molH2����ʱv�� ___v��(ѡ����>����<������=��)��
��ƽ����ϵ��HCN�����ʵ���(n)��ij�������仯������ͼ��ʾ(ͼ��x��L�ֱ��ʾ�¶Ȼ�ѹǿ)��

��xΪ�¶ȣ������ߣ�____(ѡ����L1������L2��)����ȷ��ʾn(HCN)���¶ȵĹ�ϵ��
��xΪѹǿ��������____(ѡ����L1������L2��)����ȷ��ʾn(HCN)��ѹǿ�Ĺ�ϵ��
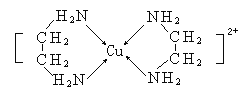
��3��NH3�ܹ��γ�Ag(NH3)2+��
��֪��Һ�д���Ag+(aq)+2NH3(aq)==Ag(NH3)2+(aq)�������¸÷�Ӧƽ�ⳣ��K1=1.10��107����ӦAgCl(s)+2NH3(aq) ![]() Ag(NH3)2+(aq) +Cl-(aq)�Ļ�ѧƽ�ⳣ��K2=1.936��10-3����Ksp(AgCl)=____________��
Ag(NH3)2+(aq) +Cl-(aq)�Ļ�ѧƽ�ⳣ��K2=1.936��10-3����Ksp(AgCl)=____________��
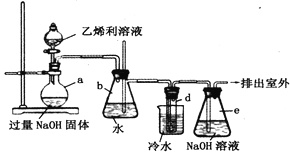
��4����������͵��������dz����Ĵ�����Ⱦ�������ͼ��ʾװ��(�缫��Ϊ���Ե缫)������SO2�����������ų�����Һ����NO2��

���缫A�ĵ缫��ӦʽΪ__________________________________��
���ڼ��������£��������ų�����Һ����NO2��ʹ��ת��Ϊ�����壬ͬʱ��SO32-���ɡ��÷�Ӧ���ӷ���ʽΪ________________________________��
���𰸡� N2H4(l)+O2(g)=N2(g)+2H2O(l) ��H= - 622.2kJ/mol 0.25mol/(L��min) < L1 L2 1.76��10-10 SO2+2H2O-2e-=SO42-+4H+ 2NO2+8OH��+4S2O42��=N2+8SO32��+4H2O
����������1����֪����H2(g)+1/2O2(g)= H2O(l)��H1=-285.8kJ/mol��N2(g)+2H2(g) = N2H4(l)��H2=+50.6kJ/mol�����ݸ�˹���ɣ��١�2-����N2H4(l)+O2(g)=N2(g)+2H2O(l)��H=-622.2kJ/mol����Ϊ��N2H4(l)+O2(g)=N2(g)+2H2O(l)��H=-622.2kJ/mol
��2����4min�ﵽƽ��ʱ������CH4�����ʵ���Ϊn(CH4)=1mol��66.7%���ɷ�Ӧ����ʽ��֪������H2�����ʵ���Ϊn(H2)=3n(CH4)= 1mol��66.7%��3=2mol��0-4min�ȣ���H2��ʾ�ĸ÷�Ӧ����v(H2)= ![]() =0.25mol/(L��min)�����ݷ�Ӧ����ʽ��������������֪���ﵽƽ��ʱc(CH4)=
=0.25mol/(L��min)�����ݷ�Ӧ����ʽ��������������֪���ﵽƽ��ʱc(CH4)= ![]() mol/L ��c(NH3)=
mol/L ��c(NH3)= ![]() mol/L��c(HCN)=
mol/L��c(HCN)= ![]() mol/L ��c(H2)=1mol/L ����ƽ�ⳣ��
mol/L ��c(H2)=1mol/L ����ƽ�ⳣ��![]() ������ƽ���������г���2molNH3��2molH2��
������ƽ���������г���2molNH3��2molH2��![]() ��˵��v��<v����ƽ�������ƶ���
��˵��v��<v����ƽ�������ƶ���
���÷�ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ���HCN�����ʵ���������L1����ȷ��ʾn(HCN)���¶ȵĹ�ϵ���÷�ӦΪ���������������ķ�Ӧ������ѹǿƽ�������ƶ���HCN�����ʵ�����С����L2����ȷ��ʾn(HCN)��ѹǿ�Ĺ�ϵ����Ϊ����0.25mol/(L��min) �� < ��L1 ��L2
��3��Ksp(AgCl)= ![]() =
=![]() =1.76��10-10 ����1.76��10-10
=1.76��10-10 ����1.76��10-10
��4������װ��ͼ����Ϣ��֪�缫AΪ�������缫��ӦʽΪSO2+2H2O-2e-=SO42-+4H+��
�������ų�����Һ�к���S2O42-����NO2����ΪSO32-�����ݵ�ʧ�����غ��ԭ���غ㣬�÷�Ӧ�����ӷ���ʽΪ2NO2+8OH-+4S2O42-=N2+8SO32-+4H2O��
������SO2+2H2O-2e-=SO42-+4H+��2NO2+8OH-+4S2O42-=N2+8SO32-+4H2O