��Ŀ����
����Ŀ��̼������Ȼ���д������ڵ�Ԫ�أ��輰�仯�����ǹ�ҵ������Ҫ�IJ��ϡ��ֹ���Ʊ��ж��ַ�����
����һ��SiO2+2C![]() Si+2CO�� ��������SiO2+2Mg
Si+2CO�� ��������SiO2+2Mg![]() Si+2MgO (�����õ������ԭ��������B-11 P-31)
Si+2MgO (�����õ������ԭ��������B-11 P-31)
��1����̬��ԭ���д���____________�������෴�ĵ�������̬Mg������������ռ�ݵ��ܼ��ĵ���������ͼ��________��
��2��������Ӧ������Ԫ�ص�һ��������С��Ԫ����________(��Ԫ�ط���)��
��3���ԱȽ�C(���ʯ)������Si��CO�������ʵ��۷е�Ӹߵ��͵�˳��___________________���Խ���ԭ��____________________��
��4��CO��������п���Ϊ���壬��Cr(CO)6���������ԭ����________(��Ԫ�ط���)1mol��������к�����������Ŀ_________��
��5��SiO2����(����ͼ)������ɽ����ʯ����(����ͼ)�е�Cԭ���û���Siԭ�ӣ�Ȼ����Si-Si֮�����Oԭ�Ӷ��γɡ�


���Ʋ�SiO2������Si����________�ӻ���O-Si-O�ļ���________________��
��SiO2�����У�����Siԭ��____________����Oԭ��______________����
��������ʯ�����ı߳�Ϊapm���Լ���þ������ܶ�_________g/cm3(д������ʽ����)��
���𰸡� 6 ���� Mg C>Si>CO ���ʯ�;���趼��ԭ�Ӿ����Ҿ���ṹ���ƣ�C��ԭ�Ӱ뾶С�ڹ��ԭ�ӣ����Խ��ʯ��C-C�����̣����ܴ����Խ��ʯ���۷е�Ⱦ����С��CO�Ƿ��Ӿ����۷е���С C 12NA Sp3 109028 8 16 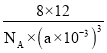
����������1����̬��ԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p2��ÿһ��s�ܼ���1�������s���ȫ������3�������෴�ĵ�����2p�ܼ���3�������ȫ�������ӣ�2P�����3�������෴�ĵ��ӣ����Ի�̬��ԭ���д���6�������෴�ĵ�����Mg�Ļ�̬��������Ų�Ϊ1s22s22p63s2��3S���������㣬S�ܼ��ĵ�����ͼ�����εģ����Ի�̬Mg ������������ռ�ݵ��ܼ��ĵ���������ͼ�����Σ�
��2����һ��������ԭ��ʧȥ������һ������������������һ��������ֵԽС��ԭ��Խ����ʧȥһ��������������Ӧ���漰��Ԫ���У�C��O��Mg��Si������C��O��Si�Ƿǽ���Ԫ�أ�����ʧȥ���ӣ� MgԪ�صĽ�������ǿ������ʧȥ���ӣ�����Ԫ�ص�һ��������С��Ԫ���ǣ�Mg��
��3��C (���ʯ)������Si����ԭ�Ӿ��壬�۵�ߣ����ǽṹ���ƣ���̼ԭ�ӵİ뾶�ȹ�ԭ�Ӱ뾶С�����ۼ��ļ��ܴ�����C (���ʯ)���۵�Ⱦ���Si�ߣ���CO�Ƿ��Ӿ��壬�۷е�ͣ������������ʵ��۷е�Ӹߵ��͵�˳��C>Si>CO��ԭ���ʯ�;���趼��ԭ�Ӿ����Ҿ���ṹ���ƣ�C��ԭ�Ӱ뾶С�ڹ��ԭ�ӣ����Խ��ʯ��C-C�����̣����ܴ����Խ��ʯ���۷е�Ⱦ����С��CO�Ƿ��Ӿ����۷е���С ��
��4�������и����¶Ե�������������ֱ���γ���λ����ԭ�ӣ�����λԭ����CO��Cr(CO)6�п���Ϊ���壬��ԭ����C��CO��N2�ǵȵ����壬��ṹʽ��C��O ��1molCO������������Ŀ��2mol������1mol��������к�����������Ŀ12NA��
��5�����ڶ������辧����ÿ����ԭ������Χ��4����ԭ�ӵijɼ��������ʯ�����е�̼ԭ������Χ4��̼ԭ�����ӵ��������ͬ�ġ�����SiO2������Si����SP3�ӻ������ʯ����������ṹ��Ԫ���������109028������SiO2������O-Si-O�ļ���Ҳ��109028��
��SiO2����Ϊ��������ṹ��ÿ��SiO2��������Siԭ�ӵĸ���Ϊ8��1/8+6��1/2+4=8����Si��Oԭ�Ӹ����ȣ�Oԭ������16��
��һ����������8��Cԭ�ӽṹ������һ������������Ϊ12��8/NA=96/NA�������߳�Ϊanm������SiO2������ܶ��ѣ�m/V��96/��NA��a��10-3��3��

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�����Ŀ��һ���¶��£�������������ĺ����ܱ������У���Ӧ2CO2(g)��6H2(g) ![]() C2H5OH(g)��3H2O(g)��ƽ�⣬����˵����ȷ����
C2H5OH(g)��3H2O(g)��ƽ�⣬����˵����ȷ����
���� | �¶�/K | ���ʵ���ʼŨ��(mol/L) | ���ʵ�ƽ��Ũ��(mol/L) | |||
CO2(g) | H2(g) | C2H5OH(g) | H2O(g) | C2H5OH(g) | ||
�� | 500 | 0.20 | 0.60 | 0 | 0 | 0.083 |
�� | 500 | 0.40 | 1.20 | 0 | 0 | |
�� | 600 | 0 | 0 | 0.10 | 0.30 | 0.039 |
A. �÷�Ӧ����ӦΪ���ȷ�Ӧ
B. ��ƽ��ʱ���������е��淴Ӧ���ʱ��������е�С
C. ��ƽ��ʱ��ת���ʣ���(CO2 ,��)����(C2H5OH ,��)��1
D. ��ƽ��ʱ���ס��������ڣ�2c(CO2 ,��)��c(CO2 ,��)