��Ŀ����
����Ŀ������ȩ(����ˮ���������л��ܼ����ܶ�Լ����ˮ���ܶ�)�ڼ��������·����绯��Ӧ�����Ʊ����״�(��ˮ���ܽ�Ȳ����������л��ܼ����ܶ�Լ����ˮ���ܶ�)�ͱ����ᡣ��Ӧԭ�����£�2C6H5CHO+NaOH��C6H5CH2OH+C6H5COONa��C6H5COONa+HC1��C6H5COOH+NaC1
������������������
����ȩ | ���״� | ������ | �� | |
�е㣯�� | 178 | 205 | 249 | 80 |
�۵㣯�� | 26 | -15 | 12 | 5.5 |
��������ˮ�е��ܽ�� | ||
17�� | 25�� | 100�� |
0.21g | 0.34g | 5.9g |
ʵ��������ͼ��

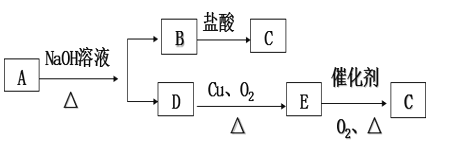
��1���ڢٲ�����������1Сʱ(��ͼ1)�����м��Ⱥ̶�װ��δ����������A������Ϊ____����������B��Ϊ����C��Ч������B��˵��ԭ��____��

��2�������ڵ�ʵ������Ϊ____��
��3���������÷�ˮԡ���������ٽ��в�����(��ͼ2)���ռ�___�����֡�ͼ2����һ�����Դ�����ȷ��Ӧ��Ϊ____��
��4������ʱ(��ͼ3)�ձ��б����ᾧ��ת�벼��©��ʱ�������ϻ�ճ���������壬��___��ϴ�����ϲ����ľ��壬������ɺ���____ϴ�Ӿ��塣
��5���õ�����ƽȷ��ȡ0.2440g��������Ʒ����ƿ�У���100mL����ˮ�ܽ�(��Ҫʱ���Լ���)������0.1000 mol��L-1�ı�NaOH��Һ�ζ���������NaOH��Һ19.20mL���ζ�ѡ�õ�ָʾ��Ϊ___����������Ʒ�Ĵ���Ϊ____%(����4λ��Ч����)��
���𰸡�������ƿ B�ĽӴ��������ȴ��������ȩ��Ч���� ��ȡ 205 �¶ȼƵ�ˮ����Ӧ����������ƿ��֧�ܿ� ��Һ ��ˮ����ˮ ��̪ 96.00
��������
��1������װ��ͼ��֪����A������������������Ϊ������ƿ����������ƿ��������BΪ���������ܣ�����CΪֱ�������ܣ�B�ĽӴ��������ȴ��������ȩ��Ч���ã�������������B��Ϊ����C��Ч������B��
�ʴ�Ϊ��������ƿ����������ƿ����B�ĽӴ��������ȴ��������ȩ��Ч���ã�
��2��A����Һ©�����в�����������ʹ��֮ǰ������Ƿ�©ˮ��ѡ��A��ȷ��
B����Һ©���ڵ�Һ�岻�ܹ��࣬����������ѡ��B��ȷ��
C������������֮ǰ��Ӧ��ʹ��Һ©�����������ϵİ��ۻ�С��©�����Ͽھ�����С�ף�ʹ�������ͨ��ѡ��C����
D����Һʱ���²��Һ�����������ر������������ձ����ӷ�Һ©���Ͽڽ��ϲ�Һ�嵹����ѡ��D����
��ѡCD��
��3�������ܵ�Ŀ���ǵõ����״�����֣������ռ�205�����֣�����ʱ���¶ȼƲ������DZ��״��������¶ȣ������¶ȼƵ�ˮ����Ӧ����������ƿ��֧�ܿڴ���
��4�����ձ��еı����ᾧ��ת�벼��©��ʱ��������������ճ���������壬��ѡ��Һ�彫�ձ����ϵľ����������ת�벼��©����Ŀ�ļ��پ������ʧ������ѡ���ϴ��Һ��Ӧ���Dz���ʹ�����ܽ���ʧ��Ҳ����������ʵģ�ѡ������Һ����ϴ����õģ�ϴ��ʱΪϴ�����壬Ӧ��ϴ�Ӽ�����ͨ����ֽ����ϴ�Ӽ��;����ֽӴ���������ɺ���������ˮ����ˮ�Ծ������ϴ�ӣ�ϴ��Ӧ��Сˮ��ͷ��
��5�����ݻ�ѧ��ӦC6H5COOH+NaOH��C6H5COONa+H2O����Ӧ����0.1000mol/LNaOH��Һ19.20mL�����ʵ���Ϊ0.1000mol/L��0.0192L=0.00192mol�������б����������=0.0192mol/L��122g/mol=2.3424g������������=![]() =96.00%��
=96.00%��

����Ŀ����1����ͭ����������ʹ�õĺϽ�֮һ����Ҫ��Zn��Cu��ɡ���һ������I1��Zn��___I1(Cu)(����ڡ���С�ڡ�)��
��2����̬Fe2+�ĵ����Ų�ʽΪ___��
��3����N��Mg��Al��Si����Ԫ���У���һ��Ԫ�صĵ������������£�
������ | I1 | I2 | I3 | I4 | �� |
I0��kJ��mol-1 | 578 | 1817 | 2745 | 11575 | �� |
���Ԫ�ص�Ԫ�ط�����___��
��4��NO3-�Ŀռ乹��__(����������)��SO42-����ԭ�ӵ��ӻ���ʽΪ___��
��5��LiAlH4���л��ϳ��г��õĻ�ԭ����LiAlH4�е������ӿռ乹����___������ԭ�ӵ��ӻ���ʽΪ___�����ݼ۲���ӶԻ������ۣ�H2S��SO2��SO3����̬�����У�����ԭ�Ӽ۲���Ӷ�����ͬ���������ӵ���___��
��6����O3���ӻ�Ϊ�ȵ������һ��������Ϊ___(�ѧʽ)��
��7��N2�����ЦҼ���м�����Ŀ��n(��)��n(��)=___��
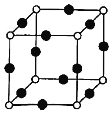
��8��Cu��N��Ԫ���γɵ�ij������ľ����ṹ��ͼ��ʾ����û�����Ļ�ѧʽ��___(��Ԫ�ط��ű�ʾ)�����������ⳤa nm�������ӵ�����ΪNA����þ�����ܶ�Ϊ___g/cm3(�ú�a��NA��ʽ�ӱ�ʾ)��
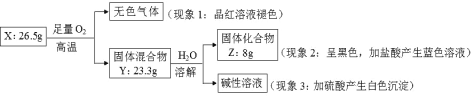
����Ŀ�����к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȡ������²��裺
(1)���ƴ���Һ����5.0g�����������ʣ����ʲ������ᷴӦ���Ĺ����ռ���Ʒ����1000mL��Һ�����ձ��Ͳ������⣬����Ҫ�IJ���������______��
(2)ѡ���̪Ϊָʾ�����еζ���
��ʢװ0.1000mol/L�������ҺӦ��ʹ��_____ʽ�ζ��ܣ�
�ڵζ�ʱ˫��Ӧ_______________��
�۵ζ��յ��ʵ������___________��
(3)�й����ݼ�¼���£�
�ⶨ��� | ������Һ�����(mL) | ���������Һ�����(mL) | |
�ζ�ǰ���� | �ζ������ | ||
�� | 20.00 | 0.50 | 20.78 |
�� | 20.00 | 1.20 | 21.32 |
���㴿�ȣ��ռ���Ʒ�Ĵ�����________����ȡ����ʵ�����������ƽ��ֵ���м��㣩
(4)������ۣ���ѡ����ƫ��������ƫ����������Ӱ������
��������ˮ��ϴ��ƿ����ʹ�ⶨ���__________��
���ڵζ������в�����������Һ������ƿ�⣬��ʹ�ⶨ���_______��
�۶���ʱ���ζ�ǰ���ӣ��ζ����ӣ���ʹ�ⶨ���__________��
��װ��Һ֮ǰ��û���ñ�Һ��ϴ�ζ��ܣ���ʹ�ⶨ���__________��