��Ŀ����
����Ŀ��ij�����л���A�ķ�����Ϊ88��̼����������Ϊ54.5%�������������Ϊ9.1%������Ϊ�����ɷ�����ͼ��ʾ�仯��
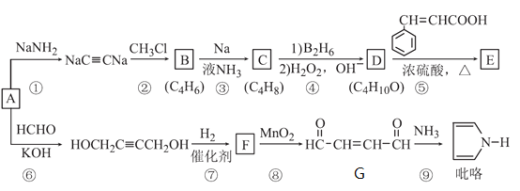
��1��C�й����ŵ�������__________ ��
��2��D��E�ķ�Ӧ������____________��
��3���л���A������������Һ��Ӧ�Ļ�ѧ����ʽΪ___________��
��4����֪ij��X����Է���������AС16��������̼���������֮��Ϊ5:1������˵����ȷ����________��
A.��ͬ������X���ܶȱ�ˮС
B. ��X��������������ԭ��Ӧ
C.��������X��Ϊͬϵ��
D.C��D��E��������������ͭ����
���𰸡��Ȼ� ������Ӧ CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH ACD
��������
�л���A�ķ�����Ϊ88��̼����������Ϊ54.5%�������������Ϊ9.1%������Ϊ��������1molA�к�̼n(C)=88g��54.5![]() ��12g/mol=4mol,����n(H)=88��9.1%��1g/mol=8mol������n(O)= 88g
��12g/mol=4mol,����n(H)=88��9.1%��1g/mol=8mol������n(O)= 88g![]() (1-54.5%
(1-54.5%![]() 9.1%)��16g/mol=2mol������A�ķ���ʽΪ��C4H8O2����A��NaOH��ˮ������AΪ������ͼ��֪BΪ�����ƣ�B�����ᷴӦ�õ�C��DΪ����D�ܷ���������Ӧ����E��E���ܷ���������Ӧ����C����B��DΪ��̼���������ƺʹ�����DΪ�Ҵ���EΪ��ȩ��CΪ�����X����Է���������AС16������X����Է�������Ϊ72��������̼���������֮��5:1,��X�ķ���ʽΪ��CmHn,����12m
9.1%)��16g/mol=2mol������A�ķ���ʽΪ��C4H8O2����A��NaOH��ˮ������AΪ������ͼ��֪BΪ�����ƣ�B�����ᷴӦ�õ�C��DΪ����D�ܷ���������Ӧ����E��E���ܷ���������Ӧ����C����B��DΪ��̼���������ƺʹ�����DΪ�Ҵ���EΪ��ȩ��CΪ�����X����Է���������AС16������X����Է�������Ϊ72��������̼���������֮��5:1,��X�ķ���ʽΪ��CmHn,����12m![]() n=72��12m/n=5,���m=5 n=12����X�ķ���ʽΪC5H12������CnH2n+2����X��������
n=72��12m/n=5,���m=5 n=12����X�ķ���ʽΪC5H12������CnH2n+2����X��������
��1��������������֪��CΪ���ᣬ����C�Ĺ�����Ϊ�Ȼ���
�����Ϊ���Ȼ���
��2��D��E�ķ�Ӧ���Ҵ��Ĵ�������Ӧ����Ӧ����Ϊ������Ӧ��
�����Ϊ��������Ӧ��
��3���ɷ�����֪�л���AΪ���������������л���A������������Һ��Ӧ�Ļ�ѧ����ʽΪ��CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
�������CH3COOCH2CH3+NaOH��CH3COONa+CH3CH2OH��
��4����X����Է���������AС16������X����Է�������Ϊ72��������̼���������֮��Ϊ5:1,��X�ķ���ʽΪ��CmHn,����12m![]() n=72��12m/n=5,���m=5 n=12����X�ķ���ʽΪC5H12������CnH2n+2����X��������A.��ͬ������������ܶȱ�ˮС,��A��ȷ��B.����Ϊ�����������ܺ������ӳɣ���B����C. ��������X��Ϊͬϵ���C��ȷ��D. C��D��E�ֱ�Ϊ���ᡢ�Ҵ�����ȩ��������������ͭ������ֱ�Ϊ��������ͭ�ܽ⡢������������ש��ɫ�������ɣ�����ͬ����������������ͭ���飬��D��ȷ��
n=72��12m/n=5,���m=5 n=12����X�ķ���ʽΪC5H12������CnH2n+2����X��������A.��ͬ������������ܶȱ�ˮС,��A��ȷ��B.����Ϊ�����������ܺ������ӳɣ���B����C. ��������X��Ϊͬϵ���C��ȷ��D. C��D��E�ֱ�Ϊ���ᡢ�Ҵ�����ȩ��������������ͭ������ֱ�Ϊ��������ͭ�ܽ⡢������������ש��ɫ�������ɣ�����ͬ����������������ͭ���飬��D��ȷ��
�������ACD��