��Ŀ����
����Ŀ����֪A��B��C��D�������ڱ��еĶ�����Ԫ�أ����ǵĺ˵������������Aԭ�ӡ�Cԭ�ӵ�L�ܲ��ж�������δ�ɶԵĵ��ӣ�C��Dͬ���塣E��F���ǵ�������Ԫ�أ�Eԭ�Ӻ�����4��δ�ɶԵ��ӣ�Fԭ�ӳ������ܲ�ֻ��1�������⣬������ܲ��Ϊȫ����������������Ϣ��գ�
��1����̬Dԭ���У�����ռ�ݵ�����ܲ����Ϊ____��D��̬ԭ�Ӻ���_____��δ�ɶԵ��ӡ�
��2��E2���ļ۲�����Ų�ͼ��_______��Fԭ�ӵĵ����Ų�ʽ��_____��
��3��A������������Ӧ��ˮ������ӽṹʽΪ____��������ԭ�Ӳ�ȡ�Ĺ���ӻ���ʽΪ____��B����̬�⻯���VSEPRģ��Ϊ_____��
��4��������AC2��B2C��������DAB����Ϊ�ȵ����壬���ǽṹ���ƣ�DAB���ĽṹʽΪ____��
��5����������ɫ��Ӧ����ɫ�����ڽ�����������E3������λ��AB�����ɣ���λ��Ϊ6����ˮ��Һ��������ʵ������E2���Ķ��Լ��飬����E2�������ӷ���ʽΪ______��
���𰸡�M 2 ![]() 1s22s22p63s23p63d104s1��[Ar]3d104s1
1s22s22p63s23p63d104s1��[Ar]3d104s1 ![]() sp2 ������ [S=C=N]�� 3Fe2����2[Fe(CN)6]3��===Fe3[Fe(CN)6]2��
sp2 ������ [S=C=N]�� 3Fe2����2[Fe(CN)6]3��===Fe3[Fe(CN)6]2��
��������
A��B��C��D�������ڱ��еĶ�����Ԫ�أ����ǵĺ˵������������Aԭ�ӡ�Cԭ�ӵ�L�ܲ��ж�������δ�ɶԵĵ��ӣ���Aԭ�Ӻ�������Ų�Ϊ1s22s22p2��Cԭ�Ӻ�������Ų�Ϊ1s22s22p4����AΪ̼Ԫ�ء�CΪ��Ԫ�أ�Bԭ����������C��O֮�䣬��BΪ��Ԫ�أ�C��Dͬ���壬��DΪSԪ�أ�E��F���ǵ�������Ԫ�أ�Eԭ�Ӻ�����4��δ�ɶԵ��ӣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d64s2����EΪFe��Fԭ�ӳ������ܲ�ֻ��1�������⣬������ܲ��Ϊȫ������Fԭ�Ӻ��������=2+8+18+1=29����FΪCuԪ�أ��ݴ˴��⡣
��1����̬Sԭ���У�����ռ�ݵ�����ܲ��ǵ����㣬����M����Ԫ�ػ�̬ԭ�Ӻ���2��δ�ɶԵ��ӣ�
��2������ԭ��������26��Fe2+���ӵļ۲�����Ų�3d6�����ӵļ۲�����Ų�ͼ��![]() ��ͭ��ԭ��������29��ԭ�ӵĵ����Ų�ʽ��[Ar]3d104s1��
��ͭ��ԭ��������29��ԭ�ӵĵ����Ų�ʽ��[Ar]3d104s1��
��3��A ������������Ӧ��ˮ������̼�ᣬ���ӽṹʽΪ![]() ��������ԭ��̼ԭ���γ�2��������һ��˫������ȡ�Ĺ���ӻ���ʽΪsp2��N����̬�⻯�ﰱ�������е�ԭ�ӵļ۲���Ӷ�����4��VSEPRģ��Ϊ�����壻
��������ԭ��̼ԭ���γ�2��������һ��˫������ȡ�Ĺ���ӻ���ʽΪsp2��N����̬�⻯�ﰱ�������е�ԭ�ӵļ۲���Ӷ�����4��VSEPRģ��Ϊ�����壻
��4��������CO2��N2O��������SCN����Ϊ�ȵ����壬���ǽṹ���ƣ���˸���CO2�ĵ���ʽ���ж�SCN���ĽṹʽΪ[S=C=N]����
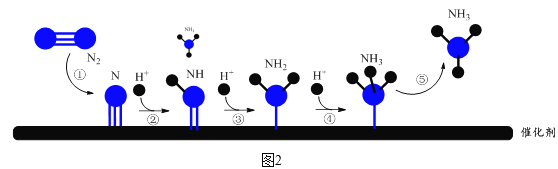
��5��һ�������軯�ؼ����������ӣ�����ʽΪ3Fe2����2[Fe(CN)6]3��=Fe3[Fe(CN)6]2����

 �Ķ��쳵ϵ�д�
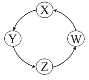
�Ķ��쳵ϵ�д�����Ŀ�����и��������У��������ͼʾ������һ�������£�һ��ת����ϵ������У� ��
��� | X | Y | Z | W |
|
�� | Si | Na2SiO3 | H2SiO3 | SiO2 | |
�� | Na | NaOH | Na2CO3 | NaCl | |
�� | Cl2 | Ca(ClO)2 | HClO | HCl | |
�� | Fe | FeCl3 | FeCl2 | Fe(OH)2 |
A.�٢ڢ�B.�٢�C.�ڢ�D.�٢�
����Ŀ�����1��A��1��B��������ѡ1������������������𣬰�1��A���֡�___
1��A�������ʼ�����;������ | 1��B�������ʼ�����;������ |
������;
| ������;
|
����Ŀ������ȩ(����ˮ���������л��ܼ����ܶ�Լ����ˮ���ܶ�)�ڼ��������·����绯��Ӧ�����Ʊ����״�(��ˮ���ܽ�Ȳ����������л��ܼ����ܶ�Լ����ˮ���ܶ�)�ͱ����ᡣ��Ӧԭ�����£�2C6H5CHO+NaOH��C6H5CH2OH+C6H5COONa��C6H5COONa+HC1��C6H5COOH+NaC1
������������������
����ȩ | ���״� | ������ | �� | |
�е㣯�� | 178 | 205 | 249 | 80 |
�۵㣯�� | 26 | -15 | 12 | 5.5 |
��������ˮ�е��ܽ�� | ||
17�� | 25�� | 100�� |
0.21g | 0.34g | 5.9g |
ʵ��������ͼ��

��1���ڢٲ�����������1Сʱ(��ͼ1)�����м��Ⱥ̶�װ��δ����������A������Ϊ____����������B��Ϊ����C��Ч������B��˵��ԭ��____��

��2�������ڵ�ʵ������Ϊ____��
��3���������÷�ˮԡ���������ٽ��в�����(��ͼ2)���ռ�___�����֡�ͼ2����һ�����Դ�����ȷ��Ӧ��Ϊ____��
��4������ʱ(��ͼ3)�ձ��б����ᾧ��ת�벼��©��ʱ�������ϻ�ճ���������壬��___��ϴ�����ϲ����ľ��壬������ɺ���____ϴ�Ӿ��塣
��5���õ�����ƽȷ��ȡ0.2440g��������Ʒ����ƿ�У���100mL����ˮ�ܽ�(��Ҫʱ���Լ���)������0.1000 mol��L-1�ı�NaOH��Һ�ζ���������NaOH��Һ19.20mL���ζ�ѡ�õ�ָʾ��Ϊ___����������Ʒ�Ĵ���Ϊ____%(����4λ��Ч����)��