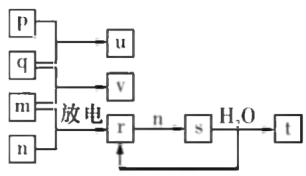
��Ŀ����
����Ŀ�����к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȡ������²��裺
(1)���ƴ���Һ����5.0g�����������ʣ����ʲ������ᷴӦ���Ĺ����ռ���Ʒ����1000mL��Һ�����ձ��Ͳ������⣬����Ҫ�IJ���������______��
(2)ѡ���̪Ϊָʾ�����еζ���
��ʢװ0.1000mol/L�������ҺӦ��ʹ��_____ʽ�ζ��ܣ�
�ڵζ�ʱ˫��Ӧ_______________��
�۵ζ��յ��ʵ������___________��
(3)�й����ݼ�¼���£�
�ⶨ��� | ������Һ�����(mL) | ���������Һ�����(mL) | |
�ζ�ǰ���� | �ζ������ | ||
�� | 20.00 | 0.50 | 20.78 |
�� | 20.00 | 1.20 | 21.32 |
���㴿�ȣ��ռ���Ʒ�Ĵ�����________����ȡ����ʵ�����������ƽ��ֵ���м��㣩
(4)������ۣ���ѡ����ƫ��������ƫ����������Ӱ������
��������ˮ��ϴ��ƿ����ʹ�ⶨ���__________��
���ڵζ������в�����������Һ������ƿ�⣬��ʹ�ⶨ���_______��
�۶���ʱ���ζ�ǰ���ӣ��ζ����ӣ���ʹ�ⶨ���__________��
��װ��Һ֮ǰ��û���ñ�Һ��ϴ�ζ��ܣ���ʹ�ⶨ���__________��
���𰸡�1000mL����ƿ�ͽ�ͷ�ι� ��ʽ ��ƿ����Һ��ɫ�仯 �������һ��������Һ����ƿ����Һ��ɫǡ���ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ 80.8% ��Ӱ�� ƫ�� ƫ�� ƫ��
��������
(1)ȷ����һ�������һ�����ʵ���Ũ�ȵ���Һ����ʹ�õIJ����������ձ�������������ͷ�ιܡ�һ����������ƿ��
(2)�����ǿ����������Ҫ����ʽ�ζ���ʢװ��������Һ�ü�ʽ�ζ���ʢװ���ζ������У�����ע����ƿ����Һ��ɫ�ı仯�����ж��ζ��յ㣬�Է�̪Ϊָʾ�����ñ�����Һ�ζ��ָʾ���ɺ�ɫ��Ϊ��ɫ��������ڲ���ɫ�ﵽ�ζ��յ㣻
(3)�ȷ������ݵ���Ч�ԣ�������������ƽ�������Ȼ����ݹ�ϵʽNaOH��HCl����������Ƶ����ʵ������ټ����ռ���Ʒ�Ĵ��ȣ�
(4)����c(����)=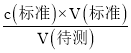 ����������������V(��)��Ӱ�죬�Դ��ж�Ũ�ȵ���
����������������V(��)��Ӱ�죬�Դ��ж�Ũ�ȵ���
(1)���Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�1000mL����ƿ�У����ò�����������ϴ���ձ��Ͳ�����2��3�Σ�ϴ��Һת�Ƶ�����ƿ�У�������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ�IJ�������Ϊ���ձ�����������1000mL����ƿ����ͷ�ιܣ�
(2)��ʢװ0.1000mol/L�������ҺӦ��ʹ����ʽ�ζ��ܣ�
�ڵζ�ʱ�۾�Ӧע��۲���ƿ����Һ��ɫ�ı仯��
���Է�̪Ϊָʾ������0.1000mol/L�������Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ����ʼʱ��ҺΪ���ԣ��Ժ�ɫ������HCl�ĵ��룬��Һ�ļ�����������ɫ��dz�����μ����һ�����ᣬ��Һ�ɺ�ɫ��Ϊ��ɫ��������ڲ��ٱ�Ϊ��ɫʱ���ζ��ﵽ�յ㡣��˵ζ��յ��ʵ�������ǵ������һ��������Һ����ƿ����Һ��ɫǡ���ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
(3)����������������ֱ�Ϊ��20.78mL-0.50mL=20.28mL��21.32mL-1.20mL=20.12mL���������ݾ���Ч�������ƽ�����Ϊ20.20mL�����ݷ���ʽ��HCl+NaOH=NaCl+H2O��֪ԭ��Һn(NaOH)=n(HCl)=![]() ��0.1000mol/L��20.20mL��10-3L/mL=0.101mol�������ռ�Ĵ���Ϊ
��0.1000mol/L��20.20mL��10-3L/mL=0.101mol�������ռ�Ĵ���Ϊ![]() ��100%=80.8%��
��100%=80.8%��
(4)��������ˮ��ϴ��ƿ�����ڲ�Ӱ��NaOH�����ʵ�������V��������Ӱ�죬��˶Բⶨ�����Ӱ�죻
���ڵζ������в�����������Һ������ƿ�⣬���V(��)ƫ����c(����)=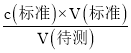 ��������֪c(����)ƫ�ߣ��Ӷ�ʹ�ⶨ���ƫ�ߣ�
��������֪c(����)ƫ�ߣ��Ӷ�ʹ�ⶨ���ƫ�ߣ�
�۶���ʱ���ζ�ǰ���ӣ��ζ����ӣ����V(��)ƫС�����ݸ���c(����)=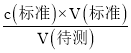 ��������֪c(����)ƫ�ͣ��Ӷ�ʹ�ⶨ���ƫ�ͣ�
��������֪c(����)ƫ�ͣ��Ӷ�ʹ�ⶨ���ƫ�ͣ�
��װ��Һ֮ǰ��û���ñ�Һ��ϴ�ζ��ܣ���ʹ�ⶨ�������ҺŨ�Ƚ��ͣ�����V(��)ƫ����c(����)=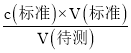 ��������֪c(����)ƫ�ߣ��Ӷ�ʹ�ⶨ���ƫ�ߡ�
��������֪c(����)ƫ�ߣ��Ӷ�ʹ�ⶨ���ƫ�ߡ�

����Ŀ������ȩ(����ˮ���������л��ܼ����ܶ�Լ����ˮ���ܶ�)�ڼ��������·����绯��Ӧ�����Ʊ����״�(��ˮ���ܽ�Ȳ����������л��ܼ����ܶ�Լ����ˮ���ܶ�)�ͱ����ᡣ��Ӧԭ�����£�2C6H5CHO+NaOH��C6H5CH2OH+C6H5COONa��C6H5COONa+HC1��C6H5COOH+NaC1
������������������
����ȩ | ���״� | ������ | �� | |
�е㣯�� | 178 | 205 | 249 | 80 |
�۵㣯�� | 26 | -15 | 12 | 5.5 |
��������ˮ�е��ܽ�� | ||
17�� | 25�� | 100�� |
0.21g | 0.34g | 5.9g |
ʵ��������ͼ��

��1���ڢٲ�����������1Сʱ(��ͼ1)�����м��Ⱥ̶�װ��δ����������A������Ϊ____����������B��Ϊ����C��Ч������B��˵��ԭ��____��

��2�������ڵ�ʵ������Ϊ____��
��3���������÷�ˮԡ���������ٽ��в�����(��ͼ2)���ռ�___�����֡�ͼ2����һ�����Դ�����ȷ��Ӧ��Ϊ____��
��4������ʱ(��ͼ3)�ձ��б����ᾧ��ת�벼��©��ʱ�������ϻ�ճ���������壬��___��ϴ�����ϲ����ľ��壬������ɺ���____ϴ�Ӿ��塣
��5���õ�����ƽȷ��ȡ0.2440g��������Ʒ����ƿ�У���100mL����ˮ�ܽ�(��Ҫʱ���Լ���)������0.1000 mol��L-1�ı�NaOH��Һ�ζ���������NaOH��Һ19.20mL���ζ�ѡ�õ�ָʾ��Ϊ___����������Ʒ�Ĵ���Ϊ____%(����4λ��Ч����)��