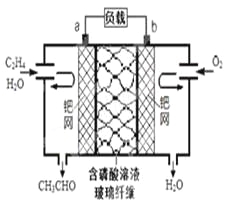
题目内容
【题目】(1)CO2是温室气体,可用NaOH溶液吸收得到Na2CO3或NaHCO3。Na2CO3俗称纯碱,已知25℃时,CO32-第一步水解的平衡常数Kh=2×10-4mol/L,当溶液中c(HCO3-):c(CO32-)=20∶ 1 时,溶液的pH=______。
(2)为了除去银器表面Ag2S,可采用如下方法:在一个铝制的容器中放入食盐溶液,将银器浸入食盐溶液,使银器与铝接触良好形成原电池.过一段时间,银器表面变为银白色,并闻到臭鸡蛋的气味,观察到有少量白色絮状沉淀生成,则原电池的正极反应为______________________________,请解释臭鸡蛋气味形成的原因(用离子方程式表示)________________________________________。

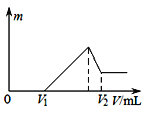
(3)25 ℃,在0.10 mol·L-1H2S溶液中,通入HCl气体或加入NaOH固体以调节溶液pH,溶液pH与c(S2-)关系如图(忽略溶液体积的变化、H2S的挥发)。
①pH=11时,溶液中的c(H2S)+c(HS-)=________mol·L-1。
②某溶液含0.040 mol·L-1M2+、0.10 mol·L-1H2S,当溶液pH=________时,Mn2+开始沉淀。[已知:Ksp(MS)=5.6×10-17]
(4)Na2S2O3溶液常作为标准液测定物质的组成。
I.取3.92 g某铁的氧化物,溶于足量稀硫酸,并配制成100.0 mL溶液;
II.取10.00 mL所得溶液,加入足量KI溶液,滴加几滴指示剂;
III.用0.2000 mol L-1的Na2S2O3标准溶液滴定,重复2~3次,平均消耗标准液20.00mL。
已知:I2+2S2O32-= S4O62-+2I-。则:
①步骤II 所用指示剂的名称为____________;判断达到滴定终点的操作和现象___________________。
②该铁的氧化物的化学式为______________。
(5)常温下,向20 mL 0.2 mol /L H2A溶液中滴加0.2 mol /L NaOH溶液。有关微粒的物质的量变化如上图,其中三条线代表的是A2-、H2A和HA-浓度变化的曲线,根据图示,当V(NaOH)=20 mL时,溶液中Na+、HA-、 A2-、 H2A四种微粒浓度大小关系:__________________________________。溶液显_______性。

【答案】 9 Ag2S +2e- = 2Ag + S2- 2Al3+ + 3S2- + 6H2O = 2Al(OH)3↓+3H2S↑ 0.0987 3 淀粉溶液 滴入最后一滴标准液时,溶液由蓝色变为无色,且半分钟内不变色 Fe5O7 Na+>HA->A2->H2A 酸性
【解析】(1)CO2是温室气体,可用NaOH溶液吸收得到Na2CO3或NaHCO3。Na2CO3俗称纯碱,已知25℃时,CO32-第一步水解的平衡常数Kh=2×10-4mol/L,当溶液中c(HCO3-):c(CO32-)=20∶1 时,由Kh=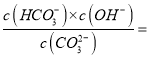 2×10-4mol/L,可以求出
2×10-4mol/L,可以求出![]() mol/L,所以
mol/L,所以![]() mol/L,溶液的pH=9。
mol/L,溶液的pH=9。
(2)为了除去银器表面Ag2S,可采用如下方法:在一个铝制的容器中放入食盐溶液,将银器浸入食盐溶液,使银器与铝接触良好形成原电池.过一段时间,银器表面变为银白色,并闻到臭鸡蛋的气味,观察到有少量白色絮状沉淀生成,则原电池的正极反应为Ag2S +2e- = 2Ag + S2-,臭鸡蛋气味形成的原因是Al3+ 和S2-发生双水解反应生成有臭鸡蛋气味的硫化氢,离子方程式为2Al3+ + 3S2- + 6H2O = 2Al(OH)3↓+3H2S↑。
(3)①由图可知,pH=11时,c(S2-)=1.3![]() mol/L。由物料守恒可得c(H2S)+c(HS-)+ c(S2-)=0.10mol·L-1,所以溶液中的c(H2S)+c(HS-)=0.10mol·L-1-1.3
mol/L。由物料守恒可得c(H2S)+c(HS-)+ c(S2-)=0.10mol·L-1,所以溶液中的c(H2S)+c(HS-)=0.10mol·L-1-1.3![]() mol/L =0.0987mol·L-1。
mol/L =0.0987mol·L-1。
②某溶液含0.040 mol·L-1M2+、0.10 mol·L-1H2S,当Mn2+开始沉淀时,c(S2-)=![]() mol/L,由图像中的信息可知,此时溶液pH=3。
mol/L,由图像中的信息可知,此时溶液pH=3。
(4)①步骤II 所用指示剂是淀粉溶液;判断达到滴定终点的操作和现象是:滴入最后一滴标准液时,溶液由蓝色变为无色,且半分钟内不变色。
②由题意可知,3.92 g某铁的氧化物溶于足量稀硫酸后,生成硫酸铁和硫酸亚铁,加入KI后,Fe3+把I-氧化为I2,然后再加入Na2S2O3标准溶液把I2还原为I-,根据电子转移得到关系式2 Fe3+~ I2~2S2O32,所以10.00 mL所得溶液中n(Fe3+)=n(S2O32)= 20.00![]() 0.2000 mol L-1=0.004000mol,则100.0 mL溶液中n(Fe3+)=0.04000mol,因此,3.92 g某铁的氧化物中n(Fe2O3)=0.02000mol,n(FeO)=
0.2000 mol L-1=0.004000mol,则100.0 mL溶液中n(Fe3+)=0.04000mol,因此,3.92 g某铁的氧化物中n(Fe2O3)=0.02000mol,n(FeO)= ![]() ,n(Fe2O3):n(FeO)=2:1,所以该铁的氧化物的化学式为Fe5O7。
,n(Fe2O3):n(FeO)=2:1,所以该铁的氧化物的化学式为Fe5O7。
(5)由图像可知,I、II、III三条线分别代表的是H2A 、HA-和A2-浓度变化的曲线,当V(NaOH)=20 mL时,H2A与NaOH恰好反应生成NaHA。由图像可知,NaHA溶液中c(A2)->c(H2A),说明的电离程度大于其水解程度,溶液显酸性,因此,Na+、HA

 轻松课堂单元测试AB卷系列答案
轻松课堂单元测试AB卷系列答案 小题狂做系列答案
小题狂做系列答案【题目】(1)一定温度下,Ksp[Mg3(PO4)2]=6.0×10-29,Ksp[Ca3(PO4)2]=6.0×10-26。向浓度均为0.20mol·L-1的MgCl2和CaCl2混合溶液中逐滴加入Na3PO4,先生成________沉淀(填化学式);当测得溶液其中一种金属阳离子沉淀完全(浓度小于10-5mol·L-1)时,溶液中的另一种金属阳离子的物质的量浓度为________。
(2)毒重石的主要成分BaCO3(含Ca2+、Mg2+、Fe3+等杂质),实验室利用毒重石制备BaCl2·2H2O的流程如下:

①毒重石用盐酸浸取前需充分研磨,目的是________。
②加入NH3·H2O调节pH=8可除去________(填离子符号),滤渣Ⅱ中含________(填化学式)。加入H2C2O4时应避免过量,原因是________。
Ca2+ | Mg2+ | Fe3+ | |
开始沉淀时的pH | 11.9 | 9.1 | 1.9 |
完全沉淀时的pH | 13.9 | 11.1 | 3.7 |
已知:Ksp(BaC2O4)=1.6×10-7,Ksp(CaC2O4)=2.3×10-9。
(3)已知25℃时,CaSO4在水中的沉淀溶解平衡曲线如图所示,向100mL该条件下的CaSO4饱和溶液中加入400mL 0.01mol·L-1 Na2SO4溶液,下列叙述正确的是___(填字母)。

A.溶液中析出CaSO4沉淀,最终溶液中c(SO42-)比原来的大
B.溶液中无沉淀析出,溶液中c(Ca2+)、c(SO42-)都变小
C.溶液中析出CaSO4沉淀,溶液中c(Ca2+)、c(SO42-)都变小
D.溶液中无沉淀析出,但最终溶液中c(SO42-)比原来的大
【题目】(1)某同学为探究酸性KMnO4溶液和H2C2O4(草酸,二元弱酸)溶液的反应过程,进行如下实验。请完成以下问题:
①写出酸性KMnO4溶液和H2C2O4的离子方程式___________________________________。
②配制100mL0.0400mol·L-1的H2C2O4溶液,除用到托盘天平、药匙、烧杯、量筒、玻璃棒等仪器外,还必须用到的玻璃仪器是_______________________________________。
③将KMnO4溶液逐滴滴入一定体积的酸性H2C2O4溶液中(温度相同,并振荡),记录的现象如下:
滴入KMnO4溶液的次序 | KMnO4溶液紫色褪去所需的时间 |
先滴入第1滴 | 60s |
褪色后,再滴入第2滴 | 15s |
褪色后,再滴入第3滴 | 3s |
褪色后,再滴入第4滴 | 1s |
请分析KMnO4溶液褪色时间变化的可能原因___________________________________。
(2)![]() 和
和![]() 在溶液中可相互转化。室温下,初始浓度为1.0 mol·L-1的Na2CrO4溶液中
在溶液中可相互转化。室温下,初始浓度为1.0 mol·L-1的Na2CrO4溶液中![]() 随c(H+)的变化如图所示
随c(H+)的变化如图所示

①用离子方程式表示溶液中![]() 和
和![]() 的转化反应_________。
的转化反应_________。
②由图可知,溶液酸性减小, ![]() 的平衡转化率_________(填“增大”“减小”或“不变”)。
的平衡转化率_________(填“增大”“减小”或“不变”)。
③升高温度,溶液中![]() 的平衡转化率减小,则该反应的ΔH_________0(填“大于”“小于”或“等于”)。
的平衡转化率减小,则该反应的ΔH_________0(填“大于”“小于”或“等于”)。