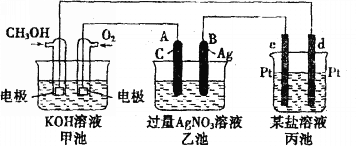
��Ŀ����
����Ŀ��ijѧ��������ʵ�飺��һ�����ڵ���KI��Һ�У���������NaClO��Һ������������ϡ���ᣬ��Һ�����������ڶ�������������ɫ��Һ�У��μ�������Na2SO3��Һ����ɫ����ʧ�������йظ�ͬѧ��ʵ��ԭ���Ľ��ͺ����ý��۵���������ȷ����(����)
A. �����ԣ�ClO����I2��SO42��
B. ��ɫ��ʧ��ԭ����Na2SO3��Һ����Ư����
C. ����KI��Һ��������ΪI����ClO������ΪI2��I2ʹ���۱���
D. ����Na2SO3��Һ������ˮ�У���ˮ��ɫ
���𰸡�B
��������
����B����ɫ��ʧ��ԭ����Na2SO3��I2��Ӧ�������˵⣬������Na2SO3��Һ����Ư���ԣ�����BΪ�������ȷѡ�A�У�����һ����ѧ��Ӧ����ʽ����ߵ������Դ����ұߵ������Կ��Եõ�
ClO��>I2>SO![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����1��ijС����������KMnO4��Һ��H2C2O4��Һ�ķ�Ӧ���˷�ӦΪ���ȷ�Ӧ����̽���������Ի�ѧ��Ӧ���ʵ�Ӱ�죬����������µķ�����¼ʵ������������Һ�������仯����
��ѡ�Լ���������0.20 mol/L H2C2O4��Һ��0.010 mol/L KMnO4��Һ�����ԣ���MnSO4������ˮ���Թܡ���Ͳ�����������ˮԡ�ۡ�
������ ��� | V��0.20 mol/L H2C2O4��Һ��/mL | V������ˮ��/mL | V��0.010 mol/L����KMnO4��Һ��/mL | m��MnSO4��/g | T/�� | �� |
�� | 2.0 | 0 | 4.0 | 0 | 50 | |
�� | 2.0 | 0 | 4.0 | 0 | 25 | |
�� | 1.5 | a | 4.0 | 0 | 25 | |
�� | 2.0 | 0 | 4.0 | 0.1 | 25 |
�ش��������⣺
��д��������Ӧ�����ӷ���ʽ��________________________��
������ʵ��٢���̽��____________�Ի�ѧ��Ӧ���ʵ�Ӱ�죻����ʵ��ڢ���̽��____________�Ի�ѧ��Ӧ����
Ӱ�졣������ʵ��ڢ���̽��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬��aΪ______________�������е���������дt/s�����������______________________��
��ʵ����ж��������ظ��������Σ�������ֵ�ֱ�13.6��13.5��13.4�����Ի��ǰ����Һ�����С�仯�����ʱ����ƽ����Ӧ����v(KMnO4)��___________ ��
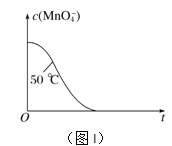
����֪ʵ���50��ʱc(MnO![]() )����Ӧʱ��t�ı仯������ͼI�������������������䣬��������ͼ�У�����ʵ���25��ʱc(MnO
)����Ӧʱ��t�ı仯������ͼI�������������������䣬��������ͼ�У�����ʵ���25��ʱc(MnO![]() )��t�ı仯����ʾ��ͼ��______________
)��t�ı仯����ʾ��ͼ��______________
 ����
����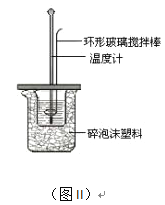
��2���ⶨ�к��ȵ�ʵ��װ����ͼII��ʾ��
��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ_______________________���к�����ֵΪ57.3 kJ/mol����
��ȡ60mL0.50mol/L NaOH��Һ��50mL0.50mol/L������Һ����ʵ�飬ʵ���������±���
ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ� ��t2-t1��/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.9 | |
2 | 26.2 | 26.4 | 26.3 | 30.6 | |
3 | 25.9 | 25.9 | 25.9 | 29.5 | |
4 | 26.4 | 26.2 | 26.3 | 30.0 | |
������Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c=4.18 J/(g����)�����к�����H=________________________kJ/mol���г�����ʽ����
���������к���ƫ�ߵ�ԭ���ǣ�����ĸ��__________��
A��ʵ��װ�ñ��¡�����Ч����
B����ȡNaOH��Һ�����ʱ���Ӷ���
C����50mL0.50mol/L���������������з�Ӧ��
D�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
E. ��ȡ������Һ�����ʱ���Ӷ���