ЬтФПФкШн
ЁОЬтФПЁПЬњМАЦфЛЏКЯЮяЪЧШеГЃЩњЛюЩњВњжагІгУЙуЗКЕФВФСЯЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЛљЬЌЬњдзгЕФМлЕчзгЙьЕРБэДяЪНЮЊ_____________________ ЁЃ
ЃЈ2ЃЉЬњдЊЫиГЃМћЕФРызггаFe2+КЭFe3+ЃЌЮШЖЈадFe2+____Fe3+(ЬюЁАДѓгкЁБЁАЁБЛђЁАаЁгкЁБ)ЃЌдвђЪЧ______________________ ЁЃ
ЃЈ3ЃЉФЩУзбѕЛЏЬњФмДпЛЏЛ№М§ЭЦНјМСNH4ClO4ЕФЗжНтЃЌNH4+ЕФНсЙЙЪНЮЊ_________________(БъГіХфЮЛМќ)ЃЌПеМфЙЙаЭЮЊ_______________ЃЌЦфжаЕЊдзгЕФдгЛЏЗНЪНЮЊ_______________ЃЛгыClO4-ЛЅЮЊЕШЕчзгЬхЕФЗжзгЛђРызгга______(ШЮаДвЛжж)ЁЃ
ЃЈ4ЃЉФГжжРызгаЭЬњЕФбѕЛЏЮяОЇАћШчЭМЫљЪОЃЌЫќгЩAЁЂBЗНПщзщГЩЁЃдђИУбѕЛЏЮяжаFe2+ЁЂFe3+ЁЂO3-ЕФИіЪ§БШЮЊ___________(ЬюзюМђећЪ§БШ)ЁЃ
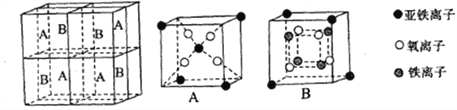
ЃЈ5ЃЉЬњгаІФЁЂІУЁЂІСШ§жжЭЌЫивьаЮЬхЃЌШчЯТЭМЫљЪОЁЃ

ІУ-FeОЇЬхЕФвЛИіОЇАћжаЫљКЌгаЕФЬњдзгЪ§ЮЊ________ЃЌІФ-FeЁЂІС-FeСНжжОЇАћжаЬњдзгЕФХфЮЛЪ§жЎБШЮЊ_________ЁЃ
вбжЊІФ-FeОЇЬхЕФУмЖШЮЊdg/cm3ЃЌNAБэЪОАЂЗќйЄЕТТоГЃЪ§ЕФЪ§жЕЃЌдђFeдзгАыОЖЮЊ_______Pm(СаБэДяЪН)ЁЃ
ЁОД№АИЁП 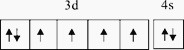 аЁгк Fe2+ЕФМлЕчзгХХВМЪНЮЊ3d6ЃЌFe3+ЕФМлЕчзгХХВМЪНЮЊ3d5ЃЌFe3+ ЕФ3dФмМЖЮЊАыТњзДЬЌНЯЮШЖЈ
аЁгк Fe2+ЕФМлЕчзгХХВМЪНЮЊ3d6ЃЌFe3+ЕФМлЕчзгХХВМЪНЮЊ3d5ЃЌFe3+ ЕФ3dФмМЖЮЊАыТњзДЬЌНЯЮШЖЈ 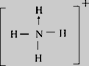 е§ЫФУцЬхаЮ sp3дгЛЏ CCl4ЁЂPO43- 1ЃК2ЃК4 4 4ЃК3
е§ЫФУцЬхаЮ sp3дгЛЏ CCl4ЁЂPO43- 1ЃК2ЃК4 4 4ЃК3 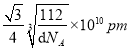
ЁОНтЮіЁПЃЈ1ЃЉЃЉFeдЊЫиЮЊ26КХдЊЫиЃЌдзгКЫЭтга26ИіЕчзгЃЌЫљвдКЫЭтЕчзгХХВМЪНЮЊЃК1s22s22p63s23p63d64s2ЃЌЛљЬЌЬњдзгЕчзгХХВМЮЊЕФМлЕчзгЙьЕРБэДяЪНЮЊ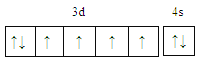 ЃЛе§ШЗД№АИЃК
ЃЛе§ШЗД№АИЃК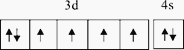 ЁЃ
ЁЃ
ЃЈ2ЃЉЬњдЊЫиГЃМћЕФРызггаFe2+КЭFe3+ЃЌЮШЖЈадFe2+аЁгкFe3+ЃЌдвђЪЧFe2+ЕФМлЕчзгХХВМЪНЮЊ3d6ЃЌFe3+ЕФМлЕчзгХХВМЪНЮЊ3d5ЃЌFe3+ЕФ3dФмМЖЮЊАыТњзДЬЌНЯЮШЖЈЃЛе§ШЗД№АИЃКаЁгк ЃЛ Fe2+ЕФМлЕчзгХХВМЪНЮЊ3d6ЃЌFe3+ЕФМлЕчзгХХВМЪНЮЊ3d5ЃЌFe3+ ЕФ3dФмМЖЮЊАыТњзДЬЌНЯЮШЖЈЁЃ
ЃЈ3ЃЉФЩУзбѕЛЏЬњФмДпЛЏЛ№М§ЭЦНјМСNH4ClO4ЕФЗжНтЃЌNH4+ЕФНсЙЙЪНЮЊ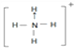 ЃЌПеМфЙЙаЭЮЊе§ЫФУцЬхаЮЃЌЦфжаЕЊдзгЕФМлВуЕчзгЖдЮЊ4ЃЌдгЛЏЗНЪНЮЊsp3дгЛЏЃЛClO4-ЪЧ5дзгЁЂ32МлЕчзгЕФРызгЃЌгыClO4-ЛЅЮЊЕШЕчзгЬхЕФЗжзгЛђРызггаCCl4ЁЂPO43-ЃЛе§ШЗД№АИЃК
ЃЌПеМфЙЙаЭЮЊе§ЫФУцЬхаЮЃЌЦфжаЕЊдзгЕФМлВуЕчзгЖдЮЊ4ЃЌдгЛЏЗНЪНЮЊsp3дгЛЏЃЛClO4-ЪЧ5дзгЁЂ32МлЕчзгЕФРызгЃЌгыClO4-ЛЅЮЊЕШЕчзгЬхЕФЗжзгЛђРызггаCCl4ЁЂPO43-ЃЛе§ШЗД№АИЃК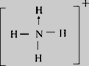 ЃЛе§ЫФУцЬхаЮ ЃЛ sp3дгЛЏ.ЃЛ CCl4ЁЂPO43- ЁЃ
ЃЛе§ЫФУцЬхаЮ ЃЛ sp3дгЛЏ.ЃЛ CCl4ЁЂPO43- ЁЃ
ЃЈ4ЃЉAКЌга1.5ИібЧЬњРызгЁЂ4ИіТШРызгЃЌBКЌга0.5ИібЧЬњРызгЁЂ4ИібѕРызгЁЂ4ИіЬњРызгЃЌдђИУбѕЛЏЮяжаFe2+ЁЂFe3+ЁЂO2-ЕФИіЪ§БШЮЊ1:2:4ЃЛе§ШЗД№АИЃК1ЃК2ЃК4ЁЃ
ЃЈ5ЃЉІУОЇЬхОЇАћжаЫљКЌгаЕФЬњдзгЪ§ЮЊ8ЁС1/8+6ЁС1/2=4ЃЌІФЁЂІССНжжОЇАћжаЬњдзгЕФХфЮЛЪ§ЗжБ№ЮЊ8ЁЂ6ЃЌдђХфЮЛЪ§жЎБШЮЊ8ЃК6=4ЃК3ЃЌгЩОЇАћЭМПЩжЊЃЌІФаЭОЇЬхЬњОЇАћжаFeдзгЪ§ФПЮЊ8ЁС1/8+1=2ЃЌОЇАћжЪСПЮЊ![]() gЃЌІФаЭОЇЬхЬњЕФУмЖШЮЊdg/cm3ЃЌОЇАћЬхЛ§ЮЊ
gЃЌІФаЭОЇЬхЬњЕФУмЖШЮЊdg/cm3ЃЌОЇАћЬхЛ§ЮЊ![]() gЁТdg/cm3=
gЁТdg/cm3=![]() cm3ЃЌдђОЇАћРтГЄ=
cm3ЃЌдђОЇАћРтГЄ=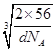 cmЃЌДІгкЬхЖдНЧЯпЩЯЕФдзгЯрСкЃЌдђ4r=
cmЃЌДІгкЬхЖдНЧЯпЩЯЕФдзгЯрСкЃЌдђ4r=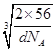 cmЁС
cmЁС![]() ЃЌЙЪr=
ЃЌЙЪr=![]() ЁС
ЁС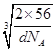 cm=
cm=![]() ЁС
ЁС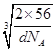 ЁС1010pmЃЌЙЪД№АИЮЊЃК4ЃЛ4ЃК3ЃЛ
ЁС1010pmЃЌЙЪД№АИЮЊЃК4ЃЛ4ЃК3ЃЛ![]() ЁС
ЁС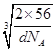 ЁС1010ЁЃе§ШЗД№АИЃК4 ЃЛ 4ЃК3ЃЛ .
ЁС1010ЁЃе§ШЗД№АИЃК4 ЃЛ 4ЃК3ЃЛ . 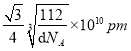 ЁЃ
ЁЃ

 УћаЃПЮЬУЯЕСаД№АИ
УћаЃПЮЬУЯЕСаД№АИ