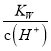جâؤ؟ؤعبف
،¾جâؤ؟،؟£¨1£©ؤ³ح¬ر§خھج½¾؟ثلذشKMnO4بـز؛؛حH2C2O4£¨²فثل£¬¶شھبُثل£©بـز؛µؤ·´س¦¹³ج£¬½ّذذبçدآتµرé،£اëحê³ةزشدآختجâ£؛
¢ظذ´³ِثلذشKMnO4بـز؛؛حH2C2O4µؤہë×س·½³جت½___________________________________،£
¢عإنضئ100mL0.0400mol،¤L-1µؤH2C2O4بـز؛£¬³سأµ½حذإججىئ½،¢ز©³×،¢ةص±،¢ء؟ح²،¢²£ء§°ôµبزائ÷ح⣬»¹±طذëسأµ½µؤ²£ء§زائ÷تا_______________________________________،£
¢غ½«KMnO4بـز؛ضًµخµخبëز»¶¨جه»µؤثلذشH2C2O4بـز؛ضذ£¨خآ¶بدàح¬£¬²¢صٌµ´£©£¬¼اآ¼µؤدضدَبçدآ£؛
µخبëKMnO4بـز؛µؤ´خذٍ | KMnO4بـز؛×دة«حتب¥ثùذèµؤت±¼ن |
دبµخبëµع1µخ | 60s |
حتة«؛َ£¬شظµخبëµع2µخ | 15s |
حتة«؛َ£¬شظµخبëµع3µخ | 3s |
حتة«؛َ£¬شظµخبëµع4µخ | 1s |
اë·ضخِKMnO4بـز؛حتة«ت±¼ن±ن»¯µؤ؟ةؤـشزٍ___________________________________،£
£¨2£©![]() ؛ح
؛ح![]() شعبـز؛ضذ؟ةد໥×ھ»¯،£تزخآدآ£¬³ُت¼إ¨¶بخھ1.0 mol،¤L-1µؤNa2CrO4بـز؛ضذ
شعبـز؛ضذ؟ةد໥×ھ»¯،£تزخآدآ£¬³ُت¼إ¨¶بخھ1.0 mol،¤L-1µؤNa2CrO4بـز؛ضذ![]() ثوc(H+)µؤ±ن»¯بçح¼ثùت¾
ثوc(H+)µؤ±ن»¯بçح¼ثùت¾

¢ظسأہë×س·½³جت½±يت¾بـز؛ضذ![]() ؛ح
؛ح![]() µؤ×ھ»¯·´س¦_________،£
µؤ×ھ»¯·´س¦_________،£
¢عسةح¼؟ةضھ£¬بـز؛ثلذش¼ُذ،£¬ ![]() µؤئ½؛â×ھ»¯آت_________£¨جî،°شِ´َ،±،°¼ُذ،،±»ٍ،°²»±ن،±£©،£
µؤئ½؛â×ھ»¯آت_________£¨جî،°شِ´َ،±،°¼ُذ،،±»ٍ،°²»±ن،±£©،£
¢غة¸كخآ¶ب£¬بـز؛ضذ![]() µؤئ½؛â×ھ»¯آت¼ُذ،£¬شٍ¸أ·´س¦µؤ¦¤H_________0£¨جî،°´َسع،±،°ذ،سع،±»ٍ،°µبسع،±£©،£
µؤئ½؛â×ھ»¯آت¼ُذ،£¬شٍ¸أ·´س¦µؤ¦¤H_________0£¨جî،°´َسع،±،°ذ،سع،±»ٍ،°µبسع،±£©،£
،¾´ً°¸،؟ ![]() 100mLبفء؟ئ؟،¢½؛ح·µخ¹ـ ·´س¦ةْ³ةµؤMn2+¶ش·´س¦سذ´ك»¯×÷سأ£¬ازMn2+µؤإ¨¶ب´َ´ك»¯ذ§¹û¸ü؛أ
100mLبفء؟ئ؟،¢½؛ح·µخ¹ـ ·´س¦ةْ³ةµؤMn2+¶ش·´س¦سذ´ك»¯×÷سأ£¬ازMn2+µؤإ¨¶ب´َ´ك»¯ذ§¹û¸ü؛أ ![]() ¼ُذ، ذ،سع
¼ُذ، ذ،سع
،¾½âخِ،؟£¨1£©¢ظ²فثلسëثلذشµؤ¸كأجثل¼ط·¢ةْرُ»¯»¹ش·´س¦£¬ةْ³ة¶رُ»¯ج¼،¢أجہë×س؛حث®£¬ہë×س·½³جت½خھ£؛2MnO4-+5H2C2O4+6H+¨T10CO2،ü+2Mn2++8H2O£»¢عإنضئ100mL0.0400mol،¤L-1µؤH2C2O4بـز؛£¬إنضئ²½ضèسذ¼ئثم،¢³ئء؟،¢بـ½â،¢ہنب´،¢زئز؛،¢د´µس،¢¶¨بف،¢ز،شبµب²ظ×÷£¬سأحذإججىئ½³ئء؟£¬سأز©³×ب،سأز©ئ·£¬شعةص±ضذبـ½â£¬ہنب´؛َ×ھزئµ½100mLبفء؟ئ؟ضذ£¬²¢سأ²£ء§°ôزء÷£¬µ±¼سث®ضءز؛أو¾àہë؟ج¶بدك1،«2cmت±£¬¸ؤسأ½؛ح·µخ¹ـµخ¼س£¬ثùزشذèزھµؤزائ÷خھ£؛حذإججىئ½،¢ء؟ح²،¢ز©³×،¢ةص±،¢²£ء§°ô،¢100mLبفء؟ئ؟،¢½؛ح·µخ¹ـ£¬¸أتشرéضذء½´خسأµ½²£ء§°ô£¬ئن×÷سأ·ض±ًتا½ء°è،¢زء÷£¬ثùزش»¹±طذëسأµ½µؤ²£ء§زائ÷تا100mLبفء؟ئ؟،¢½؛ح·µخ¹ـ£»¢غ·´س¦ةْ³ةµؤMn2+¶ش·´س¦سذ´ك»¯×÷سأ£¬ازMn2+µؤإ¨¶ب´َ´ك»¯ذ§¹û¸ü؛أ£»£¨2£©¢ظثو×إH+إ¨¶بµؤشِ´َ£¬CrO42-×ھ»¯خھCr2O72-µؤہë×س·´س¦ت½خھ£؛2CrO42-+2H+![]() Cr2O72-+H2O£»¢عبـز؛ثلذش¼ُذ،£¬ئ½؛â2CrO42-+2H+
Cr2O72-+H2O£»¢عبـز؛ثلذش¼ُذ،£¬ئ½؛â2CrO42-+2H+![]() Cr2O72-+H2Oصدٍ½ّذذ£¬CrO42-µؤئ½؛â×ھ»¯آت¼ُذ،£»¢غة¸كخآ¶ب£¬بـز؛ضذCrO42-µؤئ½؛â×ھ»¯آت¼ُذ،£¬ئ½؛âؤودٍزئ¶¯£¬ثµأ÷ص·½دٍ·إبب£¬شٍ¸أ·´س¦µؤ،÷H£¼0،£
Cr2O72-+H2Oصدٍ½ّذذ£¬CrO42-µؤئ½؛â×ھ»¯آت¼ُذ،£»¢غة¸كخآ¶ب£¬بـز؛ضذCrO42-µؤئ½؛â×ھ»¯آت¼ُذ،£¬ئ½؛âؤودٍزئ¶¯£¬ثµأ÷ص·½دٍ·إبب£¬شٍ¸أ·´س¦µؤ،÷H£¼0،£

،¾جâؤ؟،؟خھءثرذ¾؟ؤربـرخµؤ³ءµيبـ½âئ½؛â؛ح³ءµي×ھ»¯£¬ؤ³ح¬ر§ةè¼ئبçدآتµرé،£
²½ضè1£؛دٍ2 mL 0.005 mol/LAgNO3بـز؛ضذ¼سبë2 mL 0.005 mol/LKSCNبـز؛£¬¾²ضأ،£ | ³ِدض°×ة«³ءµي،£ |
²½ضè2£؛ب،1 mLةد²ماهز؛سعتش¹ـضذ£¬µخ¼س1µخ2 mol/LFe(NO3)3بـز؛،£ | بـز؛±نخھ؛ىة«،£ |
²½ضè3£؛دٍ²½ضè2µؤبـز؛ضذ£¬¼جذّ¼سبë5µخ3 mol/LAgNO3بـز؛،£ | دضدَa£¬ازبـز؛؛ىة«±نا³،£ |
²½ضè4£؛دٍ²½ضè1سàدآµؤ×از؛ضذ¼سبë5µخ3mol/L KIبـز؛،£ | ³ِدض»ئة«³ءµي،£ |
زرضھ£؛25،و£¬Ksp(AgI£¬»ئة«)= 8.3،ء1017 £¬Ksp (AgSCN£¬°×ة« )= 1.0،ء1012 ،£
»ط´ًدآءذختجâ£؛
£¨1£©²½ضè3ضذدضدَaتا________________________________،£
£¨2£©سأ³ءµيبـ½âئ½؛âشہي½âتح²½ضè4µؤتµرéدضدَ________________،£
£¨3£©دٍ50 mL 0.005 mol/LµؤAgNO3بـز؛ضذ¼سبë150 mL0.005 mol/Lµؤ KSCNبـز؛£¬بô»ى؛د؛َبـز؛جه»خھ200mL£¬شٍبـز؛ضذAg+µؤإ¨¶بش¼خھ_____mol/L ،£
،¾جâؤ؟،؟K2Cr2O7تاز»ضضضطزھµؤ»¯¹¤شءد،£زش¸ُجْ؟َ(ض÷زھ³ة·ضخھFeO،¤Cr2O3,»¹؛¬سذAl2O3،¢Fe2Oµبشسضت)خھشءدضئ±¸K2Cr2O7µؤز»ضض¹¤زصء÷³جبçدآ£؛

زرضھ£؛(a) Cr2O72-+H2O![]() 2CrO42-+2H+
2CrO42-+2H+
(b)²»ح¬خآ¶بدآ¸÷خïضتµؤبـ½â¶ب
خï ضت | KCl | NaCl | K2Cr2O7 | Na2Cr2O7 | |
بـ½â¶ب£¨g/100g H2O£© | 0،و | 28 | 35.7 | 4.7 | 163 |
40،و | 40.1 | 36.4 | 26.3 | 215 | |
80،و | 51.3 | 38 | 70 | 376 | |
(c)²½ضè¢ظµؤض÷زھ·´س¦خھ£؛FeOCr2O3+Na2CO3+NaNO3![]() Na2CrO4+Fe2O3+CO2+NaNO2£¨خ´إنئ½£©،£
Na2CrO4+Fe2O3+CO2+NaNO2£¨خ´إنئ½£©،£
£¨1£© ،°آثشü1،±؛ح ،°آثشü2،±µؤض÷زھ³ة·ض·ض±ًتا_________£¬_________(جر§ت½).
ذ´³ِ¢غµؤہë×س·´س¦·½³جت½________________________________________;
²½ضè¢ـضذµ÷PH؟ةر،سأزشدآتش¼ء__________،£
A،¢NH3 B،¢ KOH C،¢CH3COOH D،¢ HCl
£¨2£©شع²½ضè¢فضذ¼سبëتتء؟KCl£¬____________________£¬¹آثµأµ½K2Cr2O7¹ججه،£
£¨3£©ؤ³¹¤³§سأakg ¸ُجْ؟َ·غ£¨؛¬Cr2O3 40%£©ضئK2Cr2O7£¬×îضصµأµ½²ْئ· b kg£¬²ْآتخھ_____________________________،ء100%،££¨ءذ¼ئثمت½£©،£
£¨4£©»¯»¹ش·¨؟ة³ب¥·دث®ضذµؤCr2O72-£¬ب،؛¬Cr2O72-µؤؤ£ؤâث®رù·ض±ًشع²»ح¬PHجُ¼دآ,دٍأ؟¸ِث®رùضذ·ض ±ً¼سز»¶¨ء؟µؤFeSO4،¢NaHSO3£¬½ء°è£¬³ن·ض·´س¦,ب»؛َµخ¼سCa(OH)2ذü×از؛,¾²ضأ³ءµي£¬²â¶¨¸ُب¥³آت,تµرé½ل¹ûدآح¼ثùت¾،£

¢ظشعثلذشجُ¼دآ£¬NaHSO3ت¹Cr2O72-»¹ش³ةخھCr3+,اëذ´³ِNaHSO3سëCr2O72-·´س¦µؤہë×س·½³جت½:____________________________________،£
¢ع pH>8ت±£¬راجْرخ¶ش+6¼غCrµؤب¥³ذ§¹û·´¶ّدآ½µ,؟ةؤـµؤشزٍتا_________________،£