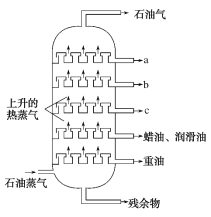
��Ŀ����
����Ŀ�����������ƣ�Na2S2O5����һ�ֳ��õĿ���������
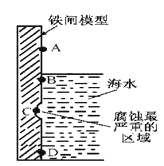
ij�о�С��Խ��������ƽ��������о���
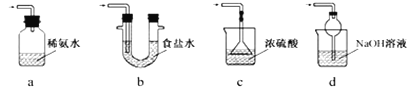
��1��������ͼװ�ã�ʵ��ǰ�ѳ���װ���ڵĿ�������ȡNa2S2O5��
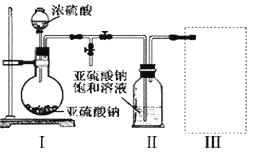
װ�â�����Na2S2O5�����������Ļ�ѧ��Ӧ����ʽΪ��Na2SO3+ SO2= Na2S2O5��
��װ�â��в�������Ļ�ѧ����ʽΪ_____________________________��
��Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽����______________��
��װ�â����ڴ���β������ѡ�õ������װ�ã��г���������ȥ��Ϊ______������ţ���
��2�����������ϣ�Na2S2O5����ˮ������NaHSO3��
��NaHSO3��Һ�����ԡ����û�ѧ����ͱ�Ҫ�����ֽ���ԭ��________________________________��
֤���ý��ۿɲ��õ�ʵ�鷽����_______________������ţ���
a���ⶨ��Һ��pH
b������Ba(OH)2��Һ
c����������
d������Ʒ����Һ
e������ɫʯ����ֽ���
�ڼ���Na2S2O5�����ڿ������ѱ�������ʵ�鷽����__________________��
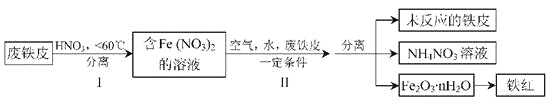
��3�����������ƣ�Na2S2O5�������������¿ɽ���ҵ��ˮ�е�Cr2O72����ԭΪCr3+��
��д���÷�Ӧ�����ӷ���ʽ_______________________��
��������Cr2O72��Ũ��Ϊ1��10-3mol/L�Ĺ�ҵ��ˮ1L������Na2S2O5����_________mg��
���𰸡�Na2SO3 + H2SO4��Ũ���� Na2SO4 + SO2��+ H2O ���� d HSO3-![]() H++SO32- HSO3-+H2O
H++SO32- HSO3-+H2O![]() H2SO3+OH-����̶ȴ���ˮ��̶ȣ�������Һ������ a��e ȡ����Na2S2O5�������Թ��У�������ˮ�ܽ⣬�μ�����ϡ���ᣬ���ٵ����Ȼ�����Һ���а�ɫ�������� ��2Cr2O72- + 3S2O52- + 10H+=4Cr3++ 6SO42- + 5H2O��2Cr2O72- +6 HSO3-+ 10H+=4Cr3++ 6SO42- + 8H2O 285
H2SO3+OH-����̶ȴ���ˮ��̶ȣ�������Һ������ a��e ȡ����Na2S2O5�������Թ��У�������ˮ�ܽ⣬�μ�����ϡ���ᣬ���ٵ����Ȼ�����Һ���а�ɫ�������� ��2Cr2O72- + 3S2O52- + 10H+=4Cr3++ 6SO42- + 5H2O��2Cr2O72- +6 HSO3-+ 10H+=4Cr3++ 6SO42- + 8H2O 285
��������
װ�â��в��������������壬װ�â��ж��������뱥�͵�����������Һ��Ӧ���ɽ��������Ƴ�����װ��III��װ��NaOH��Һ������β����������ֹ��Ⱦ������
(1)��װ�â��в�������Ϊ�����������壬��ѧ����ʽΪNa2SO3 + H2SO4��Ũ���� Na2SO4 + SO2��+ H2O��
�ڶ��������뱥�͵�����������Һ��Ӧ���ɽ��������Ƴ��������Դ�װ��II�еõ�����IJ���Ϊ���ˣ�
��װ��III�����������ն���������������ˮ�е��ܽ�Ȳ�������������������Һ���գ���ѡd��
(2)������������Һ�����ԣ�����Ϊ������������ӵĵ���̶ȴ�����ˮ��̶ȣ������ӷ���ʽ��ʾΪHSO3-![]() H++SO32-�� HSO3-+H2O
H++SO32-�� HSO3-+H2O![]() H2SO3+OH-��֤������ҺΪ���Եķ���������pH��ֽ�ⶨ��Һ��pHֵ��С��7֤����Һ�����ԣ�������ɫʯ����ֽ���飬����Һʹ��ɫʯ����ֽ���ɫ��֤����Һ�����ԣ�����������������Һ��������ɫ��������������������壬������˵����ҺΪ���ԣ�����Ʒ����Һ������֤��Һ��������أ�����ѡ��ae��
H2SO3+OH-��֤������ҺΪ���Եķ���������pH��ֽ�ⶨ��Һ��pHֵ��С��7֤����Һ�����ԣ�������ɫʯ����ֽ���飬����Һʹ��ɫʯ����ֽ���ɫ��֤����Һ�����ԣ�����������������Һ��������ɫ��������������������壬������˵����ҺΪ���ԣ�����Ʒ����Һ������֤��Һ��������أ�����ѡ��ae��
��Na2S2O5�����ڿ����б�����Ϊ�����ƣ�����֤����������ӵĴ��ڿ�֤��Na2S2O5�����ѱ�����������Ϊȡ����Na2S2O5�������Թ��У�������ˮ�ܽ⣬�μ�����ϡ���ᣬ���ٵ����Ȼ�����Һ���а�ɫ�������ɣ�
��3���ٽ��������ƣ�Na2S2O5�������������¿ɽ���ҵ��ˮ�е�Cr2O72����ԭΪCr3+������������Ϊ��������ӣ�����Ԫ���غ㣬��Ӧ����ˮ���ɣ��������ӷ���ʽΪ2Cr2O72- + 3S2O52- + 10H+=4Cr3++ 6SO42- + 5H2O��2Cr2O72- +6 HSO3-+ 10H+=4Cr3++ 6SO42- + 8H2O��
��������Cr2O72��Ũ��Ϊ1��10-3mol/L�Ĺ�ҵ��ˮ1L����Cr2O72�������ʵ�����1��10-3mol����Na2S2O5��������ʵ�����1.5��10-3mol����Na2S2O5�����������1.5��10-3mol��190g/mol=0.285g=285mg��

 �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�����Ŀ�����¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ�����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������£���֪N2(g) + 3H2(g)![]() 2NH3(g) ��H=��92.4kJ/mol����
2NH3(g) ��H=��92.4kJ/mol����
���� | �� | �� | �� |
��Ӧ��Ͷ���� | 1molN2��3molH2 | 2molNH3 | 1molNH3 |
NH3��Ũ�ȣ�mol/L�� | c1 | c2 | c3 |
��Ӧ�������仯 | �ų�a kJ | ����b kJ | ����c kJ |
��ϵѹǿ | P1 | P2 | P3 |
��Ӧ��ת���� | ��1 | ��2 | ��3 |
����˵������ȷ����(�� ��)
A.![]() ��c3B.a + b=92.4C.P2��2P3D.��1 +��3��1
��c3B.a + b=92.4C.P2��2P3D.��1 +��3��1
����Ŀ����.���úϳ�������Ҫ�ɷ�ΪCO��CO2��H2���ڴ����������ºϳɼ״�������������Ӧ���£���CO2(g)+3H2(g)![]() CH3OH��g��+H2O(g) ��H1����CO(g)+2H2(g)
CH3OH��g��+H2O(g) ��H1����CO(g)+2H2(g)![]() CH3OH(g) ��H2 ��CO2(g)+H2(g)
CH3OH(g) ��H2 ��CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H3��
CO(g)+H2O(g) ��H3��
��ѧ�� | H-H | C-O | C | H-O | C-H |
E/��kJ��mol-1�� | 436 | 343 | 1076 | 465 | X |
�ش��������⣺
��1����֪��H2=-99 kJ��mol-1��������ϱ���صĻ�ѧ�����ܣ���C![]() O����ʾCO�Ļ�ѧ��������X=_______ kJ��mol-1��
O����ʾCO�Ļ�ѧ��������X=_______ kJ��mol-1��
��2����Ӧ�١��ڡ��۶�Ӧ��ƽ�ⳣ��K1��K2��K3֮��Ĺ�ϵʽΪ___________��
��3�����ݻ�ѧ��Ӧԭ������������ѹǿ�Է�Ӧ�۵�Ӱ��Ϊ_______________������ʾ���ӶԷ�Ӧ���ʡ�ƽ��״̬��ת���ʽǶȻش�
��.�����Դ���й����Ŀ�����Ӧ��ǰ�����ɼ�С��Ⱦ����������⣬���м״������������ʵ����ȼ�ϣ�������ȼ�ϵ�ء�һ����������CO��H2�ϳ�CH3OH��CO��g��+2H2��g��![]() CH3OH��g����H=-99kJmol-1�������Ϊ2L���ܱ������г���2molCO��4molH2����ò�ͬ�¶��������ڵ�ѹǿ��P��kPa����ʱ�䣨min���ı仯��ϵ��ͼ�Т�������ʾ��
CH3OH��g����H=-99kJmol-1�������Ϊ2L���ܱ������г���2molCO��4molH2����ò�ͬ�¶��������ڵ�ѹǿ��P��kPa����ʱ�䣨min���ı仯��ϵ��ͼ�Т�������ʾ��
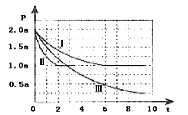
�٢�͢���ȣ��ı�ķ�Ӧ������_________��
�ڷ�Ӧ����6minʱ��ƽ�⣬�ڴ������´ӷ�Ӧ��ʼ���ﵽƽ��ʱv��CH3OH��=_________��
�۷�Ӧ����2minʱ�ﵽƽ�⣬ƽ�ⳣ��K����=_________��
�ܱȽϷ�Ӧ����¶ȣ�T1���ͷ�Ӧ����¶ȣ�T3���ĸߵͣ�T1_____T3���������������=�������жϵ�������_________��