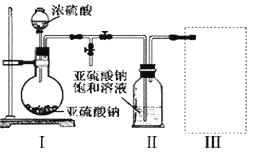
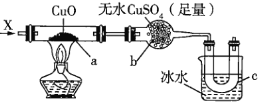
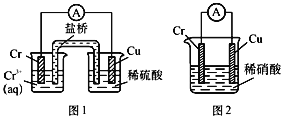
��Ŀ����
����Ŀ��������������ػ�������;�dz��㷺���ش��������⣺
��1����̬Nԭ�ӵĺ�������Ų�ʽΪ_______________ ��Crλ��Ԫ�����ڱ���������____�塣
��2��Cr��Kλ��ͬһ������������������ͬ������Ԫ��ԭ�ӵ�һ�����ܵĴ�С��ϵΪK___Cr������<������>������
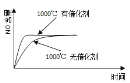
��3��CrCl3���۵㣨83�棩��CrF3���۵㣨1100�棩�͵ö࣬������Ϊ______________��
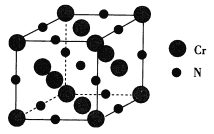
��4��Cr��һ�������ṹ��ͼ��ʾ��
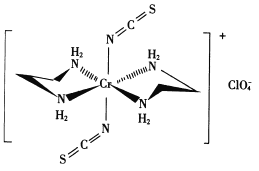
��������C1O4���Ŀռ乹��Ϊ___________�Ρ�
���������У��������ӵ���λ��Ϊ________��
��5������H2NCH2CH2NH2���Ҷ�������̼ԭ�ӵ��ӻ���ʽ��_____________������������Ԫ�ص縺�ԴӴ�С��˳��Ϊ________________��
��6�����������۵�Ϊ1770�棬����һ�־���ľ����ṹ��ͼ��ʾ�����ܶ�Ϊ5.9 g��cm��3�������ӵ�������ֵΪ6.02��1023�������ľ����߳�Ϊ_________________nm���г�����ʽ��.
���𰸡�1s22s22p3 ��B < CrCl3�Ƿ��Ӿ��壬CrF3�����Ӿ��壬���Ӽ��������������Ӽ� �������� 6 sp3 N>C>H 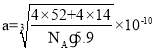
��������
��1��NԪ����7��Ԫ�أ����ݺ�������Ų����ɣ��ó���̬Nԭ�ӵĺ�������Ų���Cr��24��Ԫ�أ������ڱ�����������B�壻
��2��K��������һ������ʧȥ��CrԪ�صļ۵���Ϊ3d54s1������������ȶ����ʵ�һ������K��Cr��CrCl3���۵㣨83���������͵ķ��Ӿ������ʣ�CrF3���۵㣨1100���������͵����Ӿ������ʣ�
��3�������������C1O4-�ļ۲���Ӷ������ó���ռ乹�ͣ�
����ͼ��֪��������Cr�γɵ���λ��Ϊ6��NԪ���ṩ�µ��Ӷԣ�Cr�ṩ�չ��������N������ԭ���γɵĻ�ѧ����Ϊ��λ����
����H2NCH2CH2NH2��֪��C��Χ�γ���4�����������۲���Ӷ���Ϊ4��̼ԭ�ӵ��ӻ���ʽΪsp3�����ݵ縺�������ڱ��еı仯���ɣ�C��N��H�ĵ縺�Թ�ϵ��
��4���ɾ���ͼ�����ݾ�̯����������Cr��ԭ����Ŀ��![]() ��8+
��8+![]() ��6=4��N��ԭ����Ŀ��
��6=4��N��ԭ����Ŀ��![]() ��12+1=4�����Ծ���������m=
��12+1=4�����Ծ���������m=![]() ���ܶ���=
���ܶ���= ![]() �������Ϸ������
�������Ϸ������
��1����ԭ�ӵĻ�̬�����Ų�ʽΪ1s22s22p3������Ԫ��Ϊ24��Ԫ�أ��ʴ��ڵ������ڵ�VIB�壻
��2������Ԫ�������ɣ�ͬ����Ԫ�ش����ҵ�һ��������������˼صĵ�һ������С�ڸ���
��3�����Ȼ����Ƿ��Ӿ��壬���Ϸ��Ӿ����۷е�ϵ͵��ص㣬���������������Ӿ��壬���Ӿ�����۷е��ձ�ϴ������¼���û����Һ�����������Ӿ��壻
��4����![]() �ļ۲���Ӷ���=4��û�йµ��Ӷԣ�������ռ乹��Ϊ�������壻
�ļ۲���Ӷ���=4��û�йµ��Ӷԣ�������ռ乹��Ϊ�������壻
����ͼ���Կ�����ԭ�Ӹ�����6����ԭ�ӣ������λ��Ϊ6��
��5�����Ҷ����Ľṹ��֪̼ԭ����Χ��4�����������۲���Ӷ���Ϊ4��̼���ӻ���ʽΪsp3������ͬ����Ԫ�ش����ҵ縺������Ĺ��ɣ�̼��������ĵ縺�Թ�ϵΪ![]() ��
��
��6�����ݾ�̯�������Կ�����������4����ԭ�ӡ�4����ԭ�ӣ����Ծ���������![]() g������Ϊ�ܶ�
g������Ϊ�ܶ�![]() ����
����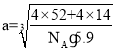 cm��
cm��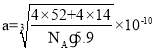 nm��
nm��