��Ŀ����
����Ŀ��H2RO3��һ�ֶ�Ԫ�ᣬ��������1L1mol��L-1Na2RO3��Һ����RO2���壬��Һ��pH��RO2��������ʵ����ı仯��ͼ��ʾ������˵����ȷ����
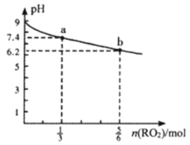
A.a����Һ��2c(Na+)=3c(RO32-)
B.��b����Һ�м�ˮ��ʹ��Һ��pH��6.2���ߵ�7.4
C.�����£�NaHRO3��Һ��c(HRO3-)>c(RO32-)>c(H2RO3)
D.������RO2����Һ������ʱ��c(Na+)=c(RO32-)+c(HRO3-)
���𰸡�C
��������
��ͼ��֪��Na2RO3��Һ�ʼ��ԣ�˵��H2RO3��һ�ֶ�Ԫ���ᣬ��1L1mol��L-1Na2RO3��Һ����RO2���壬Na2RO3��Һ��RO2���巴Ӧ������ʽ��NaHRO3����Ӧ�Ļ�ѧ����ʽΪNa2RO3+H2O+RO2=2NaHRO3��a������![]() mol RO2���壬��Һ�к���
mol RO2���壬��Һ�к���![]() mol Na2RO3��
mol Na2RO3��![]() mol NaHRO3����Һ�ʼ��ԣ�b������
mol NaHRO3����Һ�ʼ��ԣ�b������![]() mol RO2���壬��Һ�к���
mol RO2���壬��Һ�к���![]() mol Na2RO3��
mol Na2RO3��![]() mol NaHRO3����Һ�����ԡ�
mol NaHRO3����Һ�����ԡ�
A. a������![]() mol RO2���壬��Һ��Na+�����ʵ���Ϊ2mol��RO32-�����ʵ���Ϊ
mol RO2���壬��Һ��Na+�����ʵ���Ϊ2mol��RO32-�����ʵ���Ϊ![]() mol��RO32-��������Һ��ˮ�⣬��2c(Na+)>3c(RO32-)����A����
mol��RO32-��������Һ��ˮ�⣬��2c(Na+)>3c(RO32-)����A����
B. b�㵼����Һ�����ԣ�����Һ��ˮϡ�ͣ�������Ũ����С��pHֻ�����ӽ�7�����ᳬ��7����B����
C. b������![]() mol RO2���壬��Һ�к���
mol RO2���壬��Һ�к���![]() mol Na2RO3��
mol Na2RO3��![]() mol NaHRO3����Һ������˵��NaHRO3��Һ��HRO3-����̶ȴ���ˮ��̶ȣ���c(
mol NaHRO3����Һ������˵��NaHRO3��Һ��HRO3-����̶ȴ���ˮ��̶ȣ���c(![]() )>c(
)>c(![]() )>c(H2RO3)����C��ȷ��
)>c(H2RO3)����C��ȷ��
D. ������RO2����Һ������ʱ����Һ��c(H+)=c(OH-)�����ݵ���غ�c(H+)+c(Na+)=2c(RO32-)+c(HRO3-)+c(OH-)�ɵ�c(Na+)=2c(RO32-)+c(HRO3-)����D����
��ѡC��

����Ŀ��ij��ҵ��ˮ�н����±������е�5�֣�
������ |
|
������ |
|
ijͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��![]() ȡ��ˮ���������������ᣬ�ް�ɫ������������������ʹ����ʯ��ˮ����ǵ���ɫ��ζ����
ȡ��ˮ���������������ᣬ�ް�ɫ������������������ʹ����ʯ��ˮ����ǵ���ɫ��ζ����
��![]() ��������õ���Һ�м���
��������õ���Һ�м���![]() ��Һ���а�ɫ�������ɣ�
��Һ���а�ɫ�������ɣ�
�����ƶϲ���ȷ����![]()
A.��Һ��һ�����е�������![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]()
B.���м�������������ɫ��������ӷ���ʽ��![]()
C.ԭ��Һ�е�![]() ��
��![]() ��
��![]() ���������ȷ��
���������ȷ��
D.���в�����ɫ���������ӷ���ʽ��
����Ŀ����֪��(N2H4)��ǿ�������������ⳣ�������ȼ��,������ֹ��������ѺϽ���ϡ���ش���������:
��1��N2H4��H2O2�����Ԫ���е�һ������������______��
��2����Ԫ�ػ�̬ԭ�ӵĺ�������Ų�ʽΪ_____________________��
��3��1 mol N2H4�������������Լ�����ĿΪ___________��
��4��H2O2�����ֽ�ΪH2O,H2O�Ŀռ乹��Ϊ_______,������ԭ�ӵ��ӻ��������Ϊ_________��
��5��H2S��H2O2����Ҫ�����������±���ʾ:
���� | �۵�/K | �е�/K | ˮ���ܽ��(��״��) |
H2S | 187 | 202 | ÿ��ˮ���ܽ�2.6 L |
H2O2 | 272 | 423 | ������Ȼ��� |
��������۷е���ܽ�Ȳ������Ҫԭ��ֱ���_______________��________________��
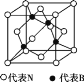
��6������þ�͵���������������زյ������β���,������:����þ_______(����ڡ���С�ڡ�)�����ơ�
��7������������ľ�����ͼ��ʾ�����ھ��������ԭ�ӵ���λ��Ϊ______���������߳�Ϊa cm,��������������ܶ���________________g��cm-3��(ֻҪ���г���ʽ,�谢���ӵ�������ֵΪNA)