��Ŀ����
20����������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ���ش��������⣺��1��ú����������Ҫ��ѧ��Ӧ����ʽΪ��C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
��2��ú�����������в������к�����H2S��Na2CO3��Һ���գ�����������ʽ�Σ��÷�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+H2S�TNaHCO3+NaHS��
��3������ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g��+CO��g��?CH3OH��g������H=-90.8kJ•mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g������H=-23.5kJ•mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g������H=-41.3kJ•mol-1
�ܷ�Ӧ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2 ��g���ġ�H=-246.4kJ•mol-1��
���ܷ�Ӧƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��ce������ĸ���ţ�
a������ b���Ӵ��� c������CO2��Ũ�� d������CO��Ũ�� e�������������
��4����֪��Ӧ��2CH3OH��g��?CH3OCH3��g��+H2O��g��ij�¶���ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH��ijʱ�̲�ø���ֵ�Ũ�������
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol/L�� | 0.44 | 0.6 | 0.6 |
��������CH3OH��10min�ﵽƽ�⣬��ʱc��CH3OH��=0.04mol/L����ʱ���ڷ�Ӧ����v��CH3OH��=0.16 mol/��L•min����
��5��2H2+CO?CH3OH�Ĵ���ΪCu2O�����о�����Ҫ��Ӧ��ϵ�ж����������CO2��ԭ���ǣ�Cu2O+CO?2Cu+CO2���û�ѧ����ʽ��ʾ����
���� ��1��ú������������ú��ˮ������������С��Ӧ������CO��H2�Ĺ��̣�
��2��������ʽ��NaHS��NaHCO3Ϊ��д��Ӧ�Ļ�ѧ����ʽ��
��3������Ȼ�ѧ����ʽ�����ø�˹���ɼ��������Ȼ�ѧ����ʽ��
��4�����ݷ���ʽ����ƽ�ⳣ����Ȼ����������ʽ�����
��5������Cu2O�ܱ�CO��ԭ����CO2��Cu���÷�ӦΪ���淴Ӧ��
��� �⣺��1��ú������������ú��ˮ������������С��Ӧ������CO��H2�Ĺ��̣�C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
�ʴ�Ϊ��C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
��2��H2S��H2CO3���Ƕ�Ԫ�ᣬ���Ƕ�������ʽ��NaHS��NaHCO3�����߷�Ӧ�Ļ�ѧ����ʽΪH2S+Na2CO3=NaHS+NaHCO3��
�ʴ�Ϊ��H2S+Na2CO3=NaHS+NaHCO3��
��3����2H2��g��+CO��g��?CH3OH��g������H=-90.8kJ•mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g������H=-23.5kJ•mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g������H=-41.3kJ•mol-1
�ɸ�˹���ɿ�֪��ͨ���١�2+��+�ۿɵ�����Ӧ����ʽ�����H=-90.8kJ/mol��2-23.5kJ/mol-41.3kJ/mol=-246.4kJ/mol��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ�Ӧʹƽ��������Ӧ�����ƶ����ɼ���CO2��Ũ�Ȼ����������ѣ����ڷ�Ӧ���ȣ������¶�ƽ�����淴Ӧ�����ƶ���ת���ʼ�С��������Ӱ��ƽ���ƶ���������
CO��Ũ�ȣ�CO��ת���ʷ�����С��
�ʴ�Ϊ��-246.4 kJ•mol-1��ce��
��4���ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ��K=$\frac{c��C{H}_{3}OC{H}_{3}��c��{H}_{2}O��}{{c}^{2}��C{H}_{3}OH��}$��������Ũ�ȴ���ƽ�ⳣ������ʽ��$\frac{0.6��0.6}{0.4{4}^{2}}$=1.86��400���ʷ�Ӧ������Ӧ������У�����Ӧ���ʴ����淴Ӧ���ʣ�
�ʴ�Ϊ������
��2CH3OH��g���PCH3OCH3��g��+H2O��g�� ijʱ��Ũ�ȣ�mol•L-1����0.44 0.6 0.6
ת��Ũ�ȣ�mol•L-1����2x x x
ƽ��Ũ�ȣ�mol•L-1����0.44-2x 0.6+x 0.6+x
K=$\frac{��0.6+x��^{2}}{��0.44-2x��^{2}}$�����x=0.2mol/L��
��ƽ��ʱc��CH3OH��=0.44mol/L-0.2mol/L��2=0.04mol/L��
��ʼʱ���ܱ������м���CH3OH��
����ʼʱ�״���Ũ��Ϊ0.44moL/L+0.6mol/L��2=1.64mol/L��ƽ��ʱc��CH3OH��=0.04mol/L��
��10minת���״�1.64moL/L-0.04moL/L=1.6mol/L��
���Լ״��ķ�Ӧ����Ϊv��CH3OH��=$\frac{1.6mol/L}{10min}$=0.16 mol/��L•min����
�ʴ�Ϊ��0.04�� 0.16��
��5�����������������Ʒ�Ӧ Cu2O+CO?2Cu+CO2������Ӧ�����ƶ���ά��Cu2O�������䣬�ʴ�Ϊ��Cu2O+CO?2Cu+CO2��
���� ���⿼���Ϊ�ۺϣ���Ŀ�Ѷ��еȣ�ע�⡰ʼ��ת��ƽ���ǽ���йػ�ѧƽ��ġ������ۡ����ⷨ����������һ��ȷ�������Խ���й�ƽ���ƽ�ⳣ�����㡢ת���ʡ���Ӧ���ʡ�ƽ��ʱ�ɷֵ���������ȵĹؼ���

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�| A�� | KSCN | B�� | NH3•H2O | C�� | NaOH��Һ | D�� | H2S��Һ |
| A�� | �������з������ͷų�����ԭ����O3�ֽ�Ĵ��� | |
| B�� | ��ѹ�ŵ�������O2����ת����O3 | |
| C�� | t��ʱ��3O2��g��?2O3��g����K��3.33��10-77 | |
| D�� | 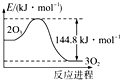 O3ת��ΪO2�������߿���ͼ��ʾ |
| A�� | ϡ��Ũ����ʱ����������Ͳ�м���һ���������ˮ��������Ͳ�ڱ�����ע��Ũ���ᣬ���ò��������Ͻ��� | |
| B�� | ����ʱ����������ĩ��Ӧ�����������ֽ��һ�ߣ�©�������ҺӦ������ֽ�ı�Ե | |
| C�� | ȼ�ŵľƾ��Ʋ�����Ӧ������ˮ��� | |
| D�� | ��ҩ��ֽ�۰ѷ�ĩ״ҩƷ���뵽�Թܵĵײ������û���ԹܼУ�������ʱ�ֳ��Թܸ�������� |
| A�� | Na+ | B�� | Cu2+ | C�� | Al3+ | D�� | Mg2+ |
| A�� | AlԪ�ر���ԭ | |
| B�� | Al2O3���������� | |
| C�� | Fe2O3�������������ǻ�ԭ�� | |
| D�� | ÿ����1molFeʱ��ת�Ƶ��ӵ����ʵ���Ϊ3mol |
| A�� | ���ά����Ҫ�ɷ��Ƕ������裬����Ҫԭ���Ƕ���������нϺõĵ����� | |
| B�� | ��±�㶹����������ˮ�뽺��������й� | |
| C�� | ���������в���H5N1�Ͳ��������ʸߣ�ͨ�����������¿�ɱ��H5N1�������в��� | |
| D�� | ������Ϊ�ִ���������Ҫ����Ⱦ֮һ������PM2.5ָ��ָ���Ƿ�ɢ��ֱ����2.5nm���� |
| A�� | ��������� | B�� | ��֬������� | C�� | ���� | D�� | ʯ̿�� |
| A�� | $\frac{14M}{{a}^{3}{N}_{A}}$ | B�� | $\frac{M}{{a}^{3}{N}_{A}}$ | C�� | $\frac{2M}{{a}^{3}{N}_{A}}$ | D�� | $\frac{4M}{{a}^{3}{N}_{A}}$ |