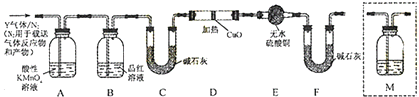
��Ŀ����
12����ѧ��������������أ�����˵������ȷ���ǣ�������| A�� | ���ά����Ҫ�ɷ��Ƕ������裬����Ҫԭ���Ƕ���������нϺõĵ����� | |
| B�� | ��±�㶹����������ˮ�뽺��������й� | |
| C�� | ���������в���H5N1�Ͳ��������ʸߣ�ͨ�����������¿�ɱ��H5N1�������в��� | |
| D�� | ������Ϊ�ִ���������Ҫ����Ⱦ֮һ������PM2.5ָ��ָ���Ƿ�ɢ��ֱ����2.5nm���� |
���� A�����ݶ�������������õĵ����Խ��
B����±�㶹����������ˮ�漰������ľ۳������ʣ�
C�������ܹ�ʹ�����ʱ��ԣ�
D��PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ����
��� �⣺A����������������õĵ����ԣ����������ά����Ҫ�ɷ֣���A��ȷ��
B����±�㶹���������˽���ľ۳������ʣ�����ˮ������ɾ��������Ե������������壬��B��ȷ��
C��ϸ������һ�ֵ����ʣ������ܹ�ʹ����ԣ�ʧȥ�������ԣ���C��ȷ��
D��PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ������D����
��ѡ��D��
���� ���⿼���˶�����������ʡ���������ʡ������ʵ����ʡ��������������Ⱦ����������ϤpM2.5�ĺ����ǽ���ؼ�����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
3���Կ��淴Ӧƽ�⣺A��g��+3B��g��?2C��g����H��0����������������ǣ�������
| A�� | �����¶ȣ�v��������v���棩������v���������ĸ��� | |
| B�� | ����ѹǿ��v��������v���棩������v���������ĸ��� | |
| C�� | ����A��Ũ�ȣ�v������������v���棩���С | |
| D�� | �����ʵ�������������v��������v���棩ͬʱ����������ı�����ͬ |
20����������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ���ش��������⣺
��1��ú����������Ҫ��ѧ��Ӧ����ʽΪ��C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
��2��ú�����������в������к�����H2S��Na2CO3��Һ���գ�����������ʽ�Σ��÷�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+H2S�TNaHCO3+NaHS��
��3������ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g��+CO��g��?CH3OH��g������H=-90.8kJ•mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g������H=-23.5kJ•mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g������H=-41.3kJ•mol-1
�ܷ�Ӧ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2 ��g���ġ�H=-246.4kJ•mol-1��
���ܷ�Ӧƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��ce������ĸ���ţ�
a������ b���Ӵ��� c������CO2��Ũ�� d������CO��Ũ�� e�������������
��4����֪��Ӧ��2CH3OH��g��?CH3OCH3��g��+H2O��g��ij�¶���ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH��ijʱ�̲�ø���ֵ�Ũ�������
�ٱȽϴ�ʱ�����淴Ӧ���ʵĴ�С��v���� v�� �����������������=������
��������CH3OH��10min�ﵽƽ�⣬��ʱc��CH3OH��=0.04mol/L����ʱ���ڷ�Ӧ����v��CH3OH��=0.16 mol/��L•min����
��5��2H2+CO?CH3OH�Ĵ���ΪCu2O�����о�����Ҫ��Ӧ��ϵ�ж����������CO2��ԭ���ǣ�Cu2O+CO?2Cu+CO2���û�ѧ����ʽ��ʾ����
��1��ú����������Ҫ��ѧ��Ӧ����ʽΪ��C+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
��2��ú�����������в������к�����H2S��Na2CO3��Һ���գ�����������ʽ�Σ��÷�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+H2S�TNaHCO3+NaHS��
��3������ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g��+CO��g��?CH3OH��g������H=-90.8kJ•mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g������H=-23.5kJ•mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g������H=-41.3kJ•mol-1
�ܷ�Ӧ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2 ��g���ġ�H=-246.4kJ•mol-1��
���ܷ�Ӧƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��ce������ĸ���ţ�
a������ b���Ӵ��� c������CO2��Ũ�� d������CO��Ũ�� e�������������
��4����֪��Ӧ��2CH3OH��g��?CH3OCH3��g��+H2O��g��ij�¶���ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH��ijʱ�̲�ø���ֵ�Ũ�������
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol/L�� | 0.44 | 0.6 | 0.6 |
��������CH3OH��10min�ﵽƽ�⣬��ʱc��CH3OH��=0.04mol/L����ʱ���ڷ�Ӧ����v��CH3OH��=0.16 mol/��L•min����
��5��2H2+CO?CH3OH�Ĵ���ΪCu2O�����о�����Ҫ��Ӧ��ϵ�ж����������CO2��ԭ���ǣ�Cu2O+CO?2Cu+CO2���û�ѧ����ʽ��ʾ����
17���������µ����Ų���ԭ�ӣ�����ӦԪ��һ������ͬһ������ǣ�������
| A�� | ���������Ų�Ϊ1s2��ԭ�Ӻ����������Ų�Ϊ2s2��ԭ�� | |
| B�� | 2p�������2��δ�ɶԵ��ӵ�ԭ�Ӻ�3p�������2��δ�ɶԵ��ӵ�ԭ�� | |
| C�� | 3p�����ֻ��1���չ����ԭ�Ӻ�4p�����ֻ��1���չ����ԭ�� | |
| D�� | �����Ų�ʽΪ1s2��ԭ�Ӻ���Χ�����Ų�ʽΪ2s22p6��ԭ�� |
4�����������м��ܸ�ϡH2SO4��Ӧ�����ܸ�����������Һ��Ӧ���ǣ�������
��NaHCO3��Al2O3��Al��OH��3 ��Al ��AlCl3��NaAlO2��
��NaHCO3��Al2O3��Al��OH��3 ��Al ��AlCl3��NaAlO2��
| A�� | �ڢۢܢ� | B�� | �ڢۢܢ� | C�� | �٢ڢۢܢ� | D�� | �٢ڢۢ� |
1�����й��ڻ�ѧ����ı�ʾ��ȷ���ǣ�������
| A�� | �������Ƶ���ʽ��Na${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$Na | |
| B�� | ������35��������45����ԭ�ӣ�${\;}_{35}^{80}$Br | |
| C�� | �����ӽṹʾ��ͼ��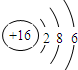 | |
| D�� | ��ϩ�Ľṹ��ʽ��C2H4 |