��Ŀ����
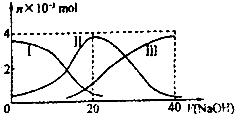 �����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ��� �仯��ͼ������I����H2A�������HA-�������A2-��������ͼʾ�жϣ�����˵����ȷ���ǣ�������
�����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ��� �仯��ͼ������I����H2A�������HA-�������A2-��������ͼʾ�жϣ�����˵����ȷ���ǣ�������| A����V��NaOH��=20mLʱ����Һ������Ũ�ȴ�С��ϵ c��Na+����c��HA-����c��H+����c��A2-����c��OH-�� |
| B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�� |
| C����ʹNaHA��Һ�����ԣ����������м������� |
| D����NaHA��Һ����ˮ�Ĺ����У�pH��������Ҳ���ܼ�С |
���㣺�����ʱ�Ķ����жϼ��й�ph�ļ���
ר�⣺����ƽ������Һ��pHר��
������A������ͼ��֪����V��NaOH��=20ʱ��������ӦΪNaOH+H2A=NaHA+H2O����Һ��ҪΪNaHA��c��A2-����c��H2A����HA-�ĵ���̶ȴ���HA-��ˮ��̶ȣ���Һ�����ԣ�
B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����ΪNaHA������ͼ���֪c��A2-����c��H2A������Һ��ʾ���ԣ�Ȼ�������Һ����������ˮ���룬�����������ӵ���ˮ��ٽ�ˮ��������жϣ�
C��NaHA��Һ��ʾ���ԣ���ʹ��Һ��ʾ���ԣ���Ҫ����Һ�н������Һ�����ܼ����
D������ͼ���֪��NaHA��Һ��ʾ���ԣ���NaHA��Һ����ˮ�Ĺ����У������̶���������Һ��������Ũ�ȼ�С����Һ��pHһ������
B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����ΪNaHA������ͼ���֪c��A2-����c��H2A������Һ��ʾ���ԣ�Ȼ�������Һ����������ˮ���룬�����������ӵ���ˮ��ٽ�ˮ��������жϣ�
C��NaHA��Һ��ʾ���ԣ���ʹ��Һ��ʾ���ԣ���Ҫ����Һ�н������Һ�����ܼ����
D������ͼ���֪��NaHA��Һ��ʾ���ԣ���NaHA��Һ����ˮ�Ĺ����У������̶���������Һ��������Ũ�ȼ�С����Һ��pHһ������
���
�⣺A����V��NaOH��=20 mLʱ��������ӦΪNaOH+H2A=NaHA+H2O����Һ������ΪNaHA������c��A2-����c��H2A��������HA-����̶ȴ���ˮ�⣬��Һ�����ԣ�����Һ������Ũ�ȴ�СΪ��c��Na+����c��HA-����c��H+����c��A2-����c��OH-������A��ȷ��
B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ���ͼʾ��ϵ֪��c��A2-����c��H2A����˵���������ˮ��̶ȣ�c��H+����c��OH-������Һ�����ԣ���Һ��������������ˮ�ĵ��룬��Һ��ˮ�ĵ���̶ȱȴ�ˮС����B����
C����ͼʾ��ϵ֪��NaHA��Һ��c��A2-����c��H2A������Һ��ʾ���ԣ����ʹ��Һ�����ԣ�Ӧ������Һ�м������Һ�����ܼ�������Һ����C����
D��NaHA��Һ����ˮ�Ĺ����У�HA-����̶���������Һ��������Ũ�ȼ�С����Һ��pH����D����
��ѡA��
B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ���ͼʾ��ϵ֪��c��A2-����c��H2A����˵���������ˮ��̶ȣ�c��H+����c��OH-������Һ�����ԣ���Һ��������������ˮ�ĵ��룬��Һ��ˮ�ĵ���̶ȱȴ�ˮС����B����
C����ͼʾ��ϵ֪��NaHA��Һ��c��A2-����c��H2A������Һ��ʾ���ԣ����ʹ��Һ�����ԣ�Ӧ������Һ�м������Һ�����ܼ�������Һ����C����
D��NaHA��Һ����ˮ�Ĺ����У�HA-����̶���������Һ��������Ũ�ȼ�С����Һ��pH����D����
��ѡA��
���������⿼���������Һ�����жϡ���Һ������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ����������ѧ���ķ��������Ŀ��飬Ϊ�߿��������ͣ���ȷͼ���������ʱ��Һ�е����ʳɷ��ǽ����Ĺؼ���ץסͼ����з������ɣ�

��ϰ��ϵ�д�
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A���ǽ���Ԫ�أ�ϡ������Ԫ�س��⣩��������Ԫ�أ������ڷ�Ӧ�ж�ֻ���������� |
| B�������£�1mol����������ϡNaOH��Һ��ȫ��Ӧת��1mol���� |
| C���ڼ��������£����Ҵ���ȥ���������е����� |
| D������ͭ��Һ�����Ե�ԭ��Cu2++2H2O�TCu��OH��2��+2H+ |
��Ԫ�������ֺ��أ�16O��17O��18O����������Ȼ������ռ��ԭ�Ӹ����ٷֱȷֱ�Ϊ��a%��b%��c%��������˵����ȷ���ǣ�������
| A��һ��16Oԭ�ӵ�����Ϊ16 g | ||
| B����Ԫ�صĽ������ԭ������Ϊ��16a%+17b%+18c%�� | ||
C����Ԫ�صĽ������ԭ������Ϊ��
| ||
| D����Ԫ�ص����ԭ������Ϊ��16a%+17b%+18c%�� |
һ�������£���Ӧ2AB��g��?A2��g��+B2��g���ﵽƽ��״̬�ı�־�ǣ�������
| A����λʱ��������n mol A2��ͬʱ����n mol B2 |
| B�������ڣ�AB��A2��B2�������干�� |
| C��AB���������ʵ���A2���������� |
| D�������и���ֵ������������ʱ��仯 |
��60g�ɼ������ϩ��ɵĻ������ͨ��ʢ��������ˮ�������ʢ��ˮ������������������28g����ԭ������м������ϩ�����ʵ���֮��Ϊ��������
| A��1��2 | B��2��1 |
| C��3��2 | D��2��3 |
һ�������£����淴Ӧ�ﵽƽ��ʱ��������
| A������ֵ�Ũ�Ȳ��ٱ仯 |
| B�����淴Ӧ���ʾ�Ϊ�� |
| C����Ӧ��Ũ��С���������Ũ�� |
| D����Ӧֹͣ�� |
��֪��H2SO3��Ka1=1.3��10-2��Ka2=6.3��10-8��H2CO3��Ka1=4.2��10-7��Ka2=5.6��10-11���ֽ���״����2.24L��CO2��2.24L��SO2�ֱ�ͨ������150mL 1mol/LNaOH��Һ�У���������Һ�ıȽ�����˵����ȷ���ǣ�������
| A��c��HCO3-����c��CO32-�� |
| B��c��HCO3-����c��HSO3-�� |
| C��c��CO32-��+c��HCO3-���Tc��SO32-��+c��HSO3-�� |
| D������Һ����ʹ���Ը��������Һ��ɫ |
 ��֪��N2O4��g��?2NO2��g����H=+57.20kJ?mol-1��
��֪��N2O4��g��?2NO2��g����H=+57.20kJ?mol-1��