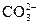��Ŀ����
��ͼ��ʵ������ȡCl2���Դ����������Cl2Ϊԭ�Ͻ����ض���Ӧ��ʵ��װ��ͼ��
��1��д��ʵ������ȡ���������ӷ�Ӧ����ʽ�� ��
��2��C��ʢװ��Һ���� ��D��ʢװ��Һ���� ��
��3��ʵ�鿪ʼ�ȵ�ȼA���ľƾ��ƣ�������K����Cl2��������װ�ã��ٵ�ȼE���ľƾ��ơ�Eװ����ʢ�����ۣ�д��Eװ���з�Ӧ�Ļ�ѧ����ʽ�� ��
 ��4��E����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е������� ��B�������� ��
��4��E����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е������� ��B�������� ��
��5����װ�õ�����Ƿ���� ����ǡ��� ���������������������Ը��� ��

��1��д��ʵ������ȡ���������ӷ�Ӧ����ʽ�� ��
��2��C��ʢװ��Һ���� ��D��ʢװ��Һ���� ��
��3��ʵ�鿪ʼ�ȵ�ȼA���ľƾ��ƣ�������K����Cl2��������װ�ã��ٵ�ȼE���ľƾ��ơ�Eװ����ʢ�����ۣ�д��Eװ���з�Ӧ�Ļ�ѧ����ʽ�� ��
 ��4��E����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е������� ��B�������� ��
��4��E����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е������� ��B�������� ����5����װ�õ�����Ƿ���� ����ǡ��� ���������������������Ը��� ��
 ��1��MnO2 + 4H+ + 2Cl-(Ũ)
��1��MnO2 + 4H+ + 2Cl-(Ũ)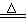 Mn2+ + Cl2��+ 2H2O
Mn2+ + Cl2��+ 2H2O��2�������Ȼ�����Һ��Ũ���� ��3��2Fe + 3Cl2 = 2FeCl3
��4��B��Һ���½�,����©����Һ������B��Һ���Ϸ��л���ɫ���壻����ʹ��ѹ���ͣ��䵱����ƿ��
��5����
 ����NaOH��Һ����β��
����NaOH��Һ����β����

��ϰ��ϵ�д�
�����Ŀ
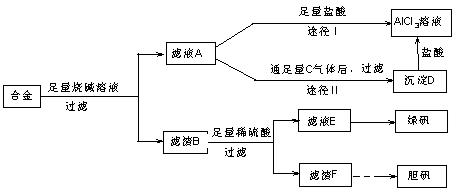
 ��
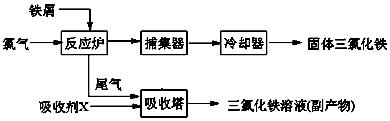
��
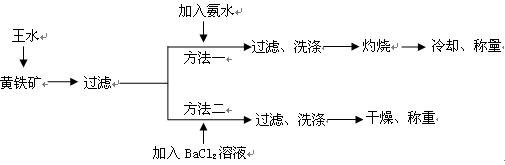
 ��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�
��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�