��Ŀ����
��15�֣��Ȼ����dz�����ˮ����������ˮFeCl3���۵�Ϊ555K���е�Ϊ588K����ҵ���Ʊ���ˮFeCl3��һ�ֹ������£�

��1����д���������з�Ӧ�����ӷ���ʽ��____________________________��
��2����֪��ˮ���Ȼ�����ˮ�е��ܽ�����£�
��FeCl3��Һ�л��FeCl3��6H2O�ķ����ǣ� ��
��3�����������¶ȳ���673K��������Է�������Ϊ325�����ʣ������ʵķ���ʽΪ�� ��
��4������ʱ��FeCl3��Һ�еμ�NaOH��Һ������ҺpHΪ2.7ʱ��Fe3+��ʼ����������ҺpHΪ4ʱ��c(Fe3+)= mol/L����֪��Ksp[Fe(OH)3]= 1.1��10��36����
��5��FeCl3����������ͨ�����õ������ⶨ����ȡm����ˮ�Ȼ�����Ʒ������ϡ���ᣬ��ת�Ƶ�100mL����ƿ��������ˮ���ݣ�ȡ��10mL�������Թ�����KI��Һ����ַ�Ӧ����ijһָʾ������c mol/L Na2S2O3��Һ�ζ���ȥV mL��
����֪��I2+2S2O32��=2I��+S4O62����
�ٵζ��յ�������ǣ�____________________________��
����Ʒ���Ȼ�������������Ϊ�� �� ��
��ijͬѧ��������������ʹ��������2���л�õ�FeCl3��6H2O��Ʒ������ˮ�Ȼ�����Ʒ���вⶨ��ͨ�����㷢�ֲ�Ʒ�е�������������100������ԭ������� ��

��1����д���������з�Ӧ�����ӷ���ʽ��____________________________��
��2����֪��ˮ���Ȼ�����ˮ�е��ܽ�����£�
| �¶�/�� | 0 | 10 | 20 | 30 | 50 | 80 | 100 |
| �ܽ��(g/100gH20) | 74.4 | 81.9 | 91.8 | 106.8 | 315.1 | 525.8 | 535.7 |
��3�����������¶ȳ���673K��������Է�������Ϊ325�����ʣ������ʵķ���ʽΪ�� ��
��4������ʱ��FeCl3��Һ�еμ�NaOH��Һ������ҺpHΪ2.7ʱ��Fe3+��ʼ����������ҺpHΪ4ʱ��c(Fe3+)= mol/L����֪��Ksp[Fe(OH)3]= 1.1��10��36����
��5��FeCl3����������ͨ�����õ������ⶨ����ȡm����ˮ�Ȼ�����Ʒ������ϡ���ᣬ��ת�Ƶ�100mL����ƿ��������ˮ���ݣ�ȡ��10mL�������Թ�����KI��Һ����ַ�Ӧ����ijһָʾ������c mol/L Na2S2O3��Һ�ζ���ȥV mL��
����֪��I2+2S2O32��=2I��+S4O62����
�ٵζ��յ�������ǣ�____________________________��
����Ʒ���Ȼ�������������Ϊ�� �� ��
��ijͬѧ��������������ʹ��������2���л�õ�FeCl3��6H2O��Ʒ������ˮ�Ȼ�����Ʒ���вⶨ��ͨ�����㷢�ֲ�Ʒ�е�������������100������ԭ������� ��
��1��2Fe2++Cl2=2Fe3++2Cl����2�֣�
��2�����������������Ũ��������ȴ�ᾧ��3�֣�
��3��Fe2Cl6��2�֣�
��4��1.1��10��6 ��2�֣�
��5���ٵ������һ�Σ���Һ��ɫ����ɫ��Ϊ��ɫ����2�֣�
��162.5Vc/m %��2�֣�
��FeCl3��6H2O���ʱʧȥ�˲��ֽᾧˮ��2�֣�
��2�����������������Ũ��������ȴ�ᾧ��3�֣�
��3��Fe2Cl6��2�֣�
��4��1.1��10��6 ��2�֣�
��5���ٵ������һ�Σ���Һ��ɫ����ɫ��Ϊ��ɫ����2�֣�
��162.5Vc/m %��2�֣�
��FeCl3��6H2O���ʱʧȥ�˲��ֽᾧˮ��2�֣�
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��
�� �������� ��
�������� ��
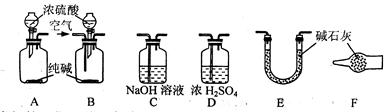
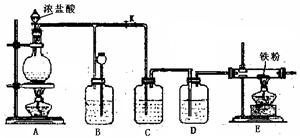
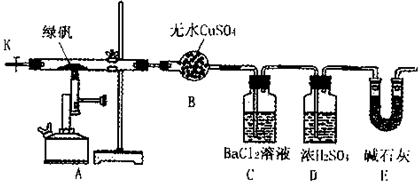
 ��4��E����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е������� ��B�������� ��
��4��E����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е������� ��B�������� �� Fe2O3+SO3��SO3��+14H2O���������������ʵ��װ������֤�̷����ȷֽ��Ƿ���������Ӧ��
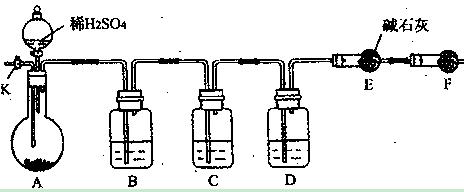
Fe2O3+SO3��SO3��+14H2O���������������ʵ��װ������֤�̷����ȷֽ��Ƿ���������Ӧ��