ЬтФПФкШн
ЁОЬтФПЁПЛЏКЯЮяFЪЧДгЮвЙњЬиВњжВЮяжаЬсШЁЕФвЛжжЩњЮяМюЃЌЦфШЫЙЄКЯГЩТЗЯпШчЭМЃК
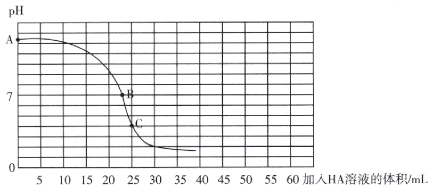
ЃЈ1ЃЉAжаКЌбѕЙйФмЭХЕФУћГЦЮЊ___КЭ___ЁЃ
ЃЈ2ЃЉCЁњDЕФЗДгІРраЭЮЊ___ЁЃ
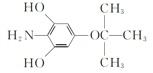
ЃЈ3ЃЉEЁњFЕФЙ§ГЬжаЃЌЛсгаИБВњЮяX(ЗжзгЪНЮЊC8H15NO2)ЩњГЩЃЌаДГіXЕФНсЙЙМђЪНЃК___ЁЃ
ЃЈ4ЃЉCЕФвЛжжЭЌЗжвьЙЙЬхЭЌЪБТњзуЯТСаЬѕМўЃЌаДГіИУЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЃК__ЁЃ
ЂйЗжзгжагаУбМќЃЛ
ЂкФмгыFeCl3ШмвКЗЂЩњЯдЩЋЗДгІЃЌФмгыбЮЫсЗДгІЃЛ
Ђлга4жжВЛЭЌЛЏбЇЛЗОГЕФЧтЧвЪ§ФПБШЮЊ9ЁУ2ЁУ2ЁУ2ЁЃ
ЃЈ5ЃЉвбжЊЃКRЁЊOЁЊRЁф+2HBr![]() RЁЊBr+RЁфЁЊBrЪЎH2O(RЁЂRЁфБэЪОЬўЛљ)ЁЃ
RЁЊBr+RЁфЁЊBrЪЎH2O(RЁЂRЁфБэЪОЬўЛљ)ЁЃ
аДГівд![]() ЁЂCH3NH2КЭCO(CH2COOH)2ЮЊдСЯжЦБИ
ЁЂCH3NH2КЭCO(CH2COOH)2ЮЊдСЯжЦБИ![]() ЕФКЯГЩТЗЯпСїГЬЭМ___(ЮоЛњЪдМСШЮгУЃЌКЯГЩТЗЯпСїГЬЭМЪОР§МћБОЬтЬтИЩ)ЁЃ
ЕФКЯГЩТЗЯпСїГЬЭМ___(ЮоЛњЪдМСШЮгУЃЌКЯГЩТЗЯпСїГЬЭМЪОР§МћБОЬтЬтИЩ)ЁЃ
ЁОД№АИЁПєЧЛљ ШЉЛљ ЛЙдЗДгІ ![]()
 Лђ
Лђ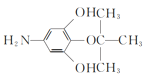
ЁОНтЮіЁП
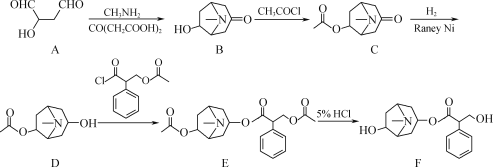
ЛЏКЯЮяAЃЈ![]() ЃЉгыCH3NH2ЁЂCO(CH2COOH)2ЗДгІЩњГЩЛЏКЯЮяBЃЈ
ЃЉгыCH3NH2ЁЂCO(CH2COOH)2ЗДгІЩњГЩЛЏКЯЮяBЃЈ![]() ЃЉЃЌBЕФєЧЛљHгыCH3COClЕФѕЃТШЗЂЩњШЁДњЃЌЩњГЩCЃЈ
ЃЉЃЌBЕФєЧЛљHгыCH3COClЕФѕЃТШЗЂЩњШЁДњЃЌЩњГЩCЃЈ![]() ЃЉЃЌCЕФєЪЛљдкRaneyNiЕФДпЛЏЯТМгH2ЛЙдЃЌЩњГЩDЃЈ
ЃЉЃЌCЕФєЪЛљдкRaneyNiЕФДпЛЏЯТМгH2ЛЙдЃЌЩњГЩDЃЈ![]() ЃЉЃЌDЕФєЧЛљHгы
ЃЉЃЌDЕФєЧЛљHгы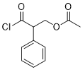 ЕФѕЃТШЗЂЩњШЁДњЃЌЩњГЩEЃЈ
ЕФѕЃТШЗЂЩњШЁДњЃЌЩњГЩEЃЈ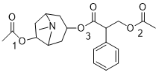 ЃЉЃЌEЕФзѓБпЃЈ1КХЮЛЃЉКЭгвБпЃЈ2КХЮЛЃЉЕФСНИіѕЅЛљдкHClЕФзїгУЯТЫЎНтЕУЕНзюжеВњЮяFЁЃ
ЃЉЃЌEЕФзѓБпЃЈ1КХЮЛЃЉКЭгвБпЃЈ2КХЮЛЃЉЕФСНИіѕЅЛљдкHClЕФзїгУЯТЫЎНтЕУЕНзюжеВњЮяFЁЃ
ЃЈ1ЃЉAжагавЛИієЧЛљЃЌСНИіШЉЛљЃЌЙЪКЌбѕЙйФмЭХЮЊєЧЛљКЭШЉЛљЃЛ
ЃЈ2ЃЉгаЛњЛЏбЇжаМгЧтЛђШЅбѕЕФЗДгІНазіЛЙдЗДгІЃЌЙЪCЁњDЕФЗДгІРраЭЮЊМгГЩЗДгІЃЛ
ЃЈ3ЃЉEЁњFЕФЙ§ГЬжаЃЌгЩгкЗДгІЬѕМўЪЧѕЅЛљЫЎНтЕФЬѕМўЃЌЛЏКЯЮяEЃЈ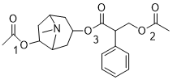 ЃЉжаМфЃЈ3ДІЃЉЕФѕЅЛљвВПЩФмЫЎНтЃЌЩњГЩ
ЃЉжаМфЃЈ3ДІЃЉЕФѕЅЛљвВПЩФмЫЎНтЃЌЩњГЩ![]() ЃЛ
ЃЛ
ЃЈ4ЃЉЂйЗжзгжагаУбМќЃЌЫЕУїЗжзгжага-O-ЃЛ
ЂкФмгыFeCl3ШмвКЗЂЩњЯдЩЋЗДгІЃЌЫЕУїгаЗгєЧЛљЃЌМДгаБНЛЗЃЛФмгыбЮЫсЗДгІЫЕУїЗжзгжагаАБЛљЃЈ-NH2ЃЉЃЛ
ЂлжЛга4жжВЛЭЌЛЏбЇЛЗОГЕФЧтЃЌЫЕУїИУЗжзгЖдГЦадНЯЧПЃЌЪ§ФПБШЮЊ9ЁУ2ЁУ2ЁУ2ЫЕУїИУЗжзгЫФжжЛЗОГЕФЧтЗжБ№ЮЊ9ЁЂ2ЁЂ2ЁЂ2ЁЃ9ИіЯрЭЌЕФЧтЃЌЫЕУїЗжзгжагаШ§Иі-CH3ЃЌШ§Иі2ЗжБ№ЪЧ-NH2ЕФЧтЃЌБНЛЗЕФЧтКЭСНИіЗг-OHЕФЧтЃЛ
злЩЯЫљЪіЃЌНсЙЙМђЪНЮЊ Лђ
Лђ ЃЛ
ЃЛ
ЃЈ5ЃЉПЩвдИљОнФцЯђКЯГЩЗЈЭЦРэЃЌИљОнЬтФПЕФСїГЬЃЌвЊЯыЕУЕН![]() ЃЌПЩвдгЩ
ЃЌПЩвдгЩ![]() гыH2ЗДгІжЦЕУЃЌ
гыH2ЗДгІжЦЕУЃЌ![]() ПЩвдгЩСїГЬжаЕФЕквЛВНЕФЗНЗЈКЯГЩЃЌгУ
ПЩвдгЩСїГЬжаЕФЕквЛВНЕФЗНЗЈКЯГЩЃЌгУ![]() ЁЂCH3NH2ЁЂCO(CH2COOH)2Ш§епЗДгІжЦЕУЃЌ
ЁЂCH3NH2ЁЂCO(CH2COOH)2Ш§епЗДгІжЦЕУЃЌ![]() гЩ
гЩ![]() ДпЛЏбѕЛЏжЦЕУЃЌ
ДпЛЏбѕЛЏжЦЕУЃЌ![]() гЩ
гЩ![]() ЫЎНтжЦЕУЃЌ
ЫЎНтжЦЕУЃЌ![]() ПЩИљОнвбжЊЕФЬсЪОЃЌгЩ
ПЩИљОнвбжЊЕФЬсЪОЃЌгЩ![]() гыHBrЗДгІжЦЕУЃЌСїГЬЮЊЃК
гыHBrЗДгІжЦЕУЃЌСїГЬЮЊЃК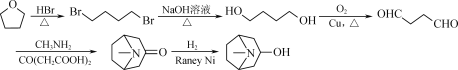

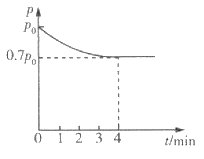
ЁОЬтФПЁПЃЈ1ЃЉдквЛЙЬЖЈШнЛ§ЕФУмБеШнЦїжаНјааШчЯТЗДгІЃКCO2(g)+H2(g)![]() CO(g)+H2O(g)ЃЌЦфЦНКтГЃЪ§KКЭЮТЖШTЕФЙиЯЕШчЯТЃК
CO(g)+H2O(g)ЃЌЦфЦНКтГЃЪ§KКЭЮТЖШTЕФЙиЯЕШчЯТЃК
T/Ёц | 700 | 800 | 850 | 1 000 | 1 200 |
K | 2.6 | 1.7 | 1.0 | 0.9 | 0.6 |
ЂйKЕФБэДяЪНЮЊ____________________ЃЛ
ЂкИУЗДгІЕФе§ЗДгІЮЊ__________ЗДгІЃЈЁАЮќШШЁБЛђЁАЗХШШЁБЃЉЃЛ
ЂлЯТСабЁЯюжаЃЌФмХаЖЯИУЗДгІвбОДяЕНЛЏбЇЦНКтзДЬЌЕФЪЧ____________(ЬюзжФИДњКХ)ЁЃ
AЃЎШнЦїжабЙЧПВЛБф BЃЎЛьКЯЦјЬхжаCOХЈЖШВЛБф
CЃЎvе§(H2)ЃНvФц(H2O) DЃЎc(CO2)ЃНc(CO)
ЃЈ2ЃЉЯТСаЛЏКЯЮяЃКЂйHClЁЁЂкNaOHЁЁЂлCH3COOHЁЁЂмNH3ЁЄH2OЁЁЂнCH3COONaЁЁЂоNH4ClЃЌШмвКГЪМюадЕФга__________(ЬюађКХ)ЃЛГЃЮТЯТ0.01 mol/L HClШмвКЕФpHЃН________ЃЛpHЃН11ЕФCH3COONaШмвКжагЩЫЎЕчРыВњЩњЕФc(OHЃ)ЃН________ЁЃ
ЃЈ3ЃЉвбжЊдкCu2ЃЋЁЂMg2ЃЋЁЂFe2ЃЋХЈЖШЯрЭЌЕФШмвКжаЃЌЦфПЊЪМГСЕэЪБЕФpHШчЯТЃК
Рызг | Fe2ЃЋ | Cu2ЃЋ | Mg2ЃЋ |
pH | 7.6 | 5.2 | 10.4 |
ЂйШєЯђИУШмвКжаЕЮМгNaOHШмвКЃЌдђЯШГСЕэЕФЪЧ ____________(ЬюРызгЗћКХ)ЃЛ
ЂкХаЖЯKsp[Mg(OH)2]____________Ksp[Fe(OH)2](ЬюЁАЃОЁБЁЂЁАЃНЁБЛђЁАЃМЁБ)ЁЃ
ЃЈ4ЃЉЕчНтзАжУШчЭМЫљЪОЃК
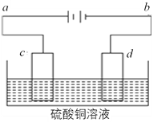
ЂйЕБгУЖшадЕчМЋЕчНтЪБЃЌdЕчМЋЕФЗДгІЪНЮЊ____________ЃЛ
ЂкШєгУДЫзАжУНјааЬњЩЯЖЦЭЃЌвбжЊЖЦВуН№ЪєВЩгУДПЭЧвЕчЖЦЧАСНЕчМЋВФСЯжЪСПЯрЕШЃЌЕчЖЦвЛЖЮЪБМфКѓЖдЕчМЋНјааГЦСПЗЂЯжСНМЋжЪСПВюЮЊ16 gЃЌдђЕчТЗжазЊвЦЕФЕчзгЮЊ____________molЁЃ