��Ŀ����
����Ŀ��25��ʱ����25 mL 0.100 0 mol L-l��BOH��Һ����ε���ͬŨ�ȵ�һԪ����HA����Һ��������ҺPH�����HA��Һ������Ĺ�ϵ������ͼ��ʾ��������˵����ȷ���ǣ� ��
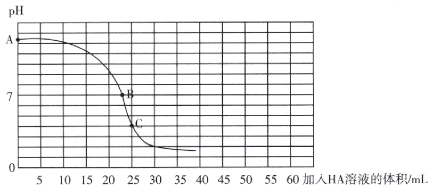
A.BOH�ĵ��뷽��ʽΪBOH =B++OH-
B.��ѡ���̪��ָʾ�����ζ��յ�ʱ����Һǡ����B�����ɫ��Ϊ�ۺ�ɫ����30���ڲ��ָ�ԭɫ
C.Ka(HA)��Kb(BOH)
D.����HA��Һ�����Ϊ50 mlʱ��c(B+)+2c(BOH)+c(OH-)=c(HA)+c(H+)
���𰸡�D
��������
A������ͼ��A���Ӧ��Һ��pH ��֪��BOHΪ�������뷽��ʽΪBOHB++OH-����A����
B������������ͼ��֪��C��ʱ���ǡ�÷�Ӧ��ȫ����Һ�����ԣ�Ӧѡ�������ָʾ�����ҷ�̪�ı�ɫ��ΧΪ8.2~10.0��B����ҺpH =7�����ڱ�ɫ��Χ�ڣ���B����
C������ͼ�ɵã�C����Һ������ΪBA����Һ�����ԣ�˵��ˮ��̶ȣ�B+��A-����Ka(HA)��Kb(BOH)����C����
D������HA��Һ�����Ϊ50 mLʱ����Һ������ΪBA��HA���Ҷ������ʵ�����ȣ��ɵ���غ㡢�����غ�ɵ� c(B+)+2c(BOH)+c(OH-)=c(HA)+c(H+)����D��ȷ��
��ѡD��

����Ŀ��ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ�Լг֡����Ⱥ;���װ�ã������ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ����
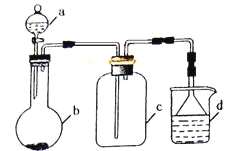
ѡ�� | a�е����� | b�е����� | c���ռ������� | d�е����� |
A | Ũ���� | Cu | NO2 | NaOH��Һ |
B | Ũ��ˮ | CaO | NH3 | H2O |
C | ϡ���� | Cu | NO | H2O |
D | Ũ���� | Cu | SO2 | ����NaHSO3��Һ |
A.AB.BC.CD.D