��Ŀ����
8�� ��ͼΪij��ѧ��ȤС����Ƶ��Ҵ�������ʵ��װ�ã�ͼ�м�������������̨�����еȾ�δ��������ͼ�У�AΪ��ˮ�Ҵ����е�Ϊ78�棩��BΪ�Ƴ�����״��ϸͭ˿����˿��CΪ��ˮCuSO4��ĩ��DΪ��ʯ�ң�FΪ���Ƶļ���Cu��OH��2����Һ��
��ͼΪij��ѧ��ȤС����Ƶ��Ҵ�������ʵ��װ�ã�ͼ�м�������������̨�����еȾ�δ��������ͼ�У�AΪ��ˮ�Ҵ����е�Ϊ78�棩��BΪ�Ƴ�����״��ϸͭ˿����˿��CΪ��ˮCuSO4��ĩ��DΪ��ʯ�ң�FΪ���Ƶļ���Cu��OH��2����Һ����ش��������⣺
��1����ͼ��ʾ���Ӻ�װ�ú��ڼ����Լ�֮ǰ��Ӧ�ȼ��������ԣ����������ƣ�
��2��������װ���У�ʵ��ʱ��Ҫ���ȵ�����Ϊ����������ij��λ�Ĵ��ţ�E��A��B��F��ΪʹA���Ҵ�ƽ�����ȵ��������Ҵ������������õIJ���������ˮԡ���ȣ�
��3��ͼ��D��ʹ�ü�ʯ�ҵ������Ƿ�ֹ���ˮ�������뵼��ʹ��ˮ����ͭ������
��4��C���۲쵽����������ˮ����ͭ������F�й۲쵽��������F�Թ����к�ɫ�������ɣ�˵��B�������ķ�Ӧ����ʽ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
���� ��1�������Լ�ǰ��Ҫȷ��װ�����������ã�
��2������ʵ���Ŀ�ĺͷ�Ӧ�������������жϣ�����ʱ����ˮԡ���ȿ������Ҵ�ƽ���������Ҵ�������
��3����ʯ�Ҿ�����ˮ���ã����Է�ֹ���ˮ�������뵼��ʹ��ˮ����ͭ������
��4��C������ˮ����ͭ����ˮ������ȩ���ܺ�������ͭ����Һ��Ӧ����ש��ɫ�������Ҵ�����������������ȩ��
��� �⣺��1������ͼʾװ�ÿ�֪����װ���к��ж���������ӣ����Լ����Լ�ǰ��Ҫ���װ�õ������ԣ�
�ʴ�Ϊ�����������ԣ�
��2��E�����������������Ҫ���ȣ�A���Ҵ���Ϊ��������ȣ�B���Ҵ��Ĵ�����Ҫ���ȣ�F��ȩ�������ķ�ӦҲҪ���ȣ�������Ҫ���ȵ�װ���У�E��A��B��F��ΪʹA���Ҵ�ƽ���������Ҵ������������õķ�����ˮԡ���ȣ�
�ʴ�Ϊ��E��A��B��F��ˮԡ���ȣ�
��3����ʯ�Ҿ�����ˮ���ã�D��ʹ�ü�ʯ�ҿ��Է�ֹF�е�ˮ��������C������ˮCuSO4���ã������Ӱ�����ˮ�ļ��飬
�ʴ�Ϊ����ֹ���ˮ�������뵼��ʹ��ˮ����ͭ������
��4��C��Ϊ��ˮ����ͭ�����ڼ����Ҵ��Ĵ�������Ӧ�����ɵ�ˮ������C�����������ȩ�ܺ�������ͭ����Һ��Ӧ������ש��ɫ����������F�л������ɫ�������Ҵ������������Ļ�ѧ����ʽΪ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
�ʴ�Ϊ����ˮ����ͭ������F�Թ����к�ɫ�������ɣ�2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
���� ���⿼�����Ҵ��Ĵ�������ʵ�鷽����Ʒ�������Ŀ�Ѷ��еȣ������������ʺ�ʵ��������������ǽ���ؼ���ע����ȷ�����л���ṹ�����ʣ�����������ѧ���ķ�����������ѧʵ��������

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�| A�� | ��������ϩ��1mol����ȫȼ�պ����ɵ�H2OΪ2mol | |
| B�� | �����£��춡����Cl2����ȡ����Ӧ���ɵ�һ�ȴ��������� | |
| C�� | �����������£�CH3CO18OC2H5��ˮ�������CH3CO18OH��C2H5OH | |
| D�� | �ú˴Ź������ײ�������HCOOCH3��HCOOCH2CH3 |
| A�� | ��ϵͳ�������� ���ƣ�4��7-����-3-�һ����� ���ƣ�4��7-����-3-�һ����� | |
| B�� | Ԫ�ط����ǿ���ͬʱ��̼���⡢������ȶ���Ԫ�ؽ��з��� | |
| C�� | ����ͬŨ�ȵ��Ҵ��ͱ���ˮ��Һ���ֱ�����ͬ�����Ľ����Ʒ�Ӧ�����Ƚ������ǻ�����Ļ��� | |
| D�� | ����������������ȼ�պ�O2������Ȳ����ϩ������ |
| A�� | NaOH | B�� | CO2 | C�� | C | D�� | SiO2 |
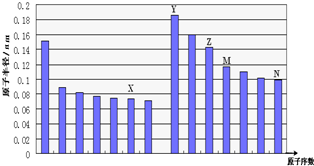
| A�� | N��Z����Ԫ�ص����Ӱ뾶���ǰ�߽ϴ� | |
| B�� | M��N����Ԫ�ص���̬�⻯����ȶ�����Ⱥ��߽�ǿ | |
| C�� | X��M����Ԫ����ɵĻ���������Ӧ�����������κ��ᷴӦ | |
| D�� | X��Y����Ԫ����ɵ�һ�ֻ����������������ӵĸ�����Ϊ1��1 |
| A�� | ���߶���ˮ�⣬��ˮ������ղ�����ͬ | |
| B�� | ���Ƕ����ã�C6H10O5��n��ʾ�����Զ���Ϊͬ���칹�� | |
| C�� | ���Ǿ��������࣬��������ζ���Ҷ��Ǹ߷��ӻ����� | |
| D�� | �����ܷ���������Ӧ������ά�ز��ܣ����ܷ���������Ӧ |

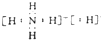 ��
�� ��
��