��Ŀ����
18����������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣺| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�أ� |
| BԪ��ԭ�ӵĺ���p��������s��������1�� |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ�I1=738kJ/mol I2=1451J/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ��������� |
| EԪ�ص������������������IJ�Ϊ4�� |
| F��ǰ�������е縺����С��Ԫ�أ� |
| G�����ڱ��ĵ����У� |
 ��
����2��B�Ļ�̬ԭ����������ߵĵ��ӣ���������ڿռ���3����չ����ԭ�ӹ���ʷĴ��Σ�
��3��ijͬѧ����������Ϣ���ƶ�C�Ļ�̬ԭ�ӵĺ�������Ų�ͼΪ��
 ����ͬѧ�����ĵ����Ų�ͼΥ��������ԭ����
����ͬѧ�����ĵ����Ų�ͼΥ��������ԭ������4��Gλ�ڢ�B��d�����۵����Ų�ʽΪ3d54s2��
��5����д��Ԫ��D��E��Fԭ�ӵ��������ӵijɶԵ�����1��3��1��
���� A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ��������������
AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�أ���AΪHԪ�أ�
BԪ��ԭ�ӵĺ���p��������s��������1��ԭ�Ӻ�������Ų�Ϊ1s22s22p3����BΪNԪ�أ�
��Cԭ�ӵĵ�һ�����ĵ��������ݿ�֪�����������ܾ�������C����+2�ۣ����ڢ�A�壬ԭ����������NԪ�أ���CΪMgԪ�أ�
D���ڵ������ڣ�Dԭ�Ӻ�������p���ȫ���������������Ų�Ϊ3s23p3����DΪPԪ�أ�
E���ڵ������ڣ�EԪ�ص������������������IJ�Ϊ4��E���ڵڢ�A�壬��EΪClԪ�أ�
F��ǰ�������е縺����С��Ԫ�أ�FΪ��������Ԫ�أ���FΪKԪ�أ�
G�ڵ����������ڱ��ĵ�7�У�GΪMnԪ�أ�
��� �⣺A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ��������������
AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�أ���AΪHԪ�أ�
BԪ��ԭ�ӵĺ���p��������s��������1��ԭ�Ӻ�������Ų�Ϊ1s22s22p3����BΪNԪ�أ�
��Cԭ�ӵĵ�һ�����ĵ��������ݿ�֪�����������ܾ�������C����+2�ۣ����ڢ�A�壬ԭ����������NԪ�أ���CΪMgԪ�أ�
D���ڵ������ڣ�Dԭ�Ӻ�������p���ȫ���������������Ų�Ϊ3s23p3����DΪPԪ�أ�
E���ڵ������ڣ�EԪ�ص������������������IJ�Ϊ4��E���ڵڢ�A�壬��EΪClԪ�أ�
F��ǰ�������е縺����С��Ԫ�أ�FΪ��������Ԫ�أ���FΪKԪ�أ�
G�ڵ����������ڱ��ĵ�7�У�GΪMnԪ�أ�
��1��NH5Ϊ���ӻ��������NH4+��H-���������ɣ�����ʽΪ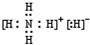 ���ʴ�Ϊ��
���ʴ�Ϊ��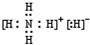 ��
��
��2��BΪNԪ�أ���������Ų�ʽΪ1s2ns2np3����̬ԭ����������ߵĵ��ӣ�����2p�ܼ�����3�����ӣ���������ڿռ���3������ԭ�ӹ���ʷĴ��Σ�
�ʴ�Ϊ��3���Ĵ���
��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ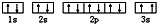 ����ͬѧ�����ĵ����Ų�ͼ��3s�ܼ���2����������������ͬ��Υ��������ԭ����
����ͬѧ�����ĵ����Ų�ͼ��3s�ܼ���2����������������ͬ��Υ��������ԭ����
�ʴ�Ϊ������ԭ����
��4��GΪMnԪ�أ���25��Ԫ�أ�λ�ڵ������ڵڢ�B�壬�������Ϊd���ӣ�Ϊd��Ԫ�أ��۵����Ų�ʽΪ3d54s2���ʴ�Ϊ����B��d��3d54s2��
��5��P��Cl��Kԭ�ӵ����������Ų��ֱ�Ϊ3s23p3��3s23p5��4s1���������ӵijɶԵ������ֱ�Ϊ1��3��1���ʴ�Ϊ��1��3��1��
���� ���⿼��ṹ������λ�ù�ϵ����������Ų����ɡ������ܡ��ӻ�������ۡ����ӽṹ�ȣ��ۺ��Խϴ��Ѷ��еȣ��ƶ�Ԫ���ǽ���Ĺؼ���ע�����֪ʶ�����գ�

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�| A�� | ��ʹʯ����Һ��ɫ | B�� | ��������NaOH��Һ��Ӧ����Na2SO4 | ||
| C�� | ������������⣨H2O2����Һ��Ӧ | D�� | ��ʹƷ����ɫ�����Ⱥ��ֳ��ֺ�ɫ |
| A�� | �кͷ�Ӧ��Ӧ�ȵIJⶨʵ���У������β�����������ɻ���ͭ˿������ⶨ�������к���ֵƫ�� | |
| B�� | ��101kPaʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285.8kJ����������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ��2H2��g��+O2��g���T2H2O��l����H=-571.6KJ/mol | |
| C�� | ��0.5molN2��1.5molH2�����ܱ������г�ַ�Ӧ����NH3��g��������19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-38.6KJ/mol | |
| D�� | HCl��NaOH��Ӧ���к��ȡ�H=-57.3KJ/mol����H2SO4��Ca��OH��2��Ӧ�ķ�Ӧ�ȡ�H=-��2��57.3��KJ/mol |
| A�� | 11��16 | B�� | 6��8 | C�� | 12��17 | D�� | 20��9 |
| A�� | 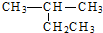 2-�һ����� 2-�һ����� | B�� | CH3CH2CH2CH2OH 1-���� | ||
| C�� | 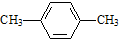 �Զ��ױ� �Զ��ױ� | D�� | 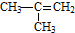 2-��-2-��ϩ 2-��-2-��ϩ |
�����뾶��r��K+����r��Al3+����r��S2-����r��Cl-��
���⻯����ȶ��ԣ�HF��HCl��H2S��PH3��SiH4
�ۻ�ԭ�ԣ�S2-��Cl-��Br-��I-
�������ԣ�Cl2��S��Se��Te
�����ԣ�H2SO4��H3PO4��H2CO3��HClO
�ǽ����ԣ�O��N��P��Si
�߽����ԣ�Be��Mg��Ca��K��
| A�� | �� | B�� | �٢� | C�� | �ڢۢܢݢޢ� | D�� | �٢ۢ� |
| A�� | +8QkJ/mol | B�� | +16Q kJ/mol | C�� | -8Q kJ/mol | D�� | -16Q kJ/mol |

| A�� | �ӻ�ȩ�ķ���ʽΪC21H26O | |
| B�� | ά����A���ӽṹ�к��б�����̼̼˫�����ǻ� | |
| C�� | �ӻ�ȩ��ά����A���������� | |
| D�� | 1 mol��1 mol����һ�������¾��������6 mol H2������Ӧ |