��Ŀ����
13����ϩ����CH2=CH-CH2OH����һ����ɫ�д̼�����ζ��Һ�壬����Ҫ���л��ϳ�ԭ�ϣ���ش���1����ϩ���ķ���ʽΪC3H6O����ϩ���к��еĹ����ŵ�������̼̼˫���ʹ��ǻ���
��2��д����ϩ����ͭ�����������·�Ӧ�Ļ�ѧ����ʽ2CH2=CH-CH2OH+O2$��_{��}^{Cu}$2CH2=CHCHO+2H2O��������˫����������
��3��д����ϩ������ˮ��Ӧ�Ļ�ѧ����ʽCH2=CH-CH2OH+Br2��CH2BrCHBrCH2OH����Ӧ����Ϊ�ӳɷ�Ӧ
��4����ϩ����CH3CO18OH����������Ӧ�Ļ�����ʽΪ��CH3CO18OH+CH2=CH-CH2OH
 CH3COOCH2CH=CH2+H218O��������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��߷��ӻ������ṹ��ʽ
CH3COOCH2CH=CH2+H218O��������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��߷��ӻ������ṹ��ʽ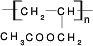 ��
��
���� ��1�����ݽṹ��ʽȷ������ʽ����ϩ���й�������̼̼˫���ʹ��ǻ���
��2����Cu�����������������£���ϩ������������Ӧ���ɱ�ϩȩ��
��3����ϩ�����巢���ӳɷ�Ӧ����1��2-���������
��4����ϩ�������ᷢ��������Ӧ���������ϩ���������ϩ����һ�������·����Ӿ۷�Ӧ���ɸ߷��ӻ����
��� �⣺��1�����ݽṹ��ʽ֪����ϩ������ʽΪC3H6O����ϩ���й�����Ϊ̼̼˫���ʹ��ǻ���
�ʴ�Ϊ��C3H6O��̼̼˫�������ǻ���
��2����Cu�����������������£���ϩ������������Ӧ���ɱ�ϩȩ����Ӧ����ʽΪ2CH2=CH-CH2OH+O2$��_{��}^{Cu}$2CH2=CHCHO+2H2O��
�ʴ�Ϊ��2CH2=CH-CH2OH+O2$��_{��}^{Cu}$2CH2=CHCHO+2H2O��
��3����ϩ������̼̼˫����������ˮ�����ӳɷ�Ӧ����Ӧ�ķ���ʽΪCH2=CH-CH2OH+Br2��CH2BrCHBrCH2OH��
�ʴ�Ϊ��CH2=CH-CH2OH+Br2��CH2BrCHBrCH2OH���ӳɷ�Ӧ��
��4��ϩ������CH3CO18OH����������Ӧ�Ļ�����ʽΪCH3CO18OH+CH2=CH-CH2OH CH3COOCH2CH=CH2+H218O��������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��۱�ϩ���������ṹ��ʽΪ
CH3COOCH2CH=CH2+H218O��������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��۱�ϩ���������ṹ��ʽΪ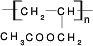 ��
��
�ʴ�Ϊ��CH3CO18OH+CH2=CH-CH2OH CH3COOCH2CH=CH2+H218O��
CH3COOCH2CH=CH2+H218O��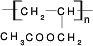 ��
��
���� ���⿼���л���ṹ�����ʣ�Ϊ��Ƶ���㣬��ȷ�����ż������ʹ�ϵ���ɽ��ע��������Ӧ�ϼ��ͳɼ���ʽ��Ϊ�״��㣮

| A�� | ���ǰ뵼����ϣ���ͬ�����Ҳ�ǰ뵼����� | |
| B�� | Ũ������Ը���HCl���壬��Ҳ����Ũ�������HI���� | |
| C�� | Na�ڿ�����ȼ�ջ�����Na2O2����Li�ڿ�����ȼ��Ҳ������Li2O2 | |
| D�� | ±��Ԫ�ص�����˵���������۵����ߣ��ʼ���������۵�Ҳ��˵�������Ӷ����� |
| A�� | ��CH2N2��n | B�� | C3H4ON5 | C�� | C3H6N6 | D�� | C4H4N5 |
| A�� | ����C��D��Ũ�� | B�� | ��СB��A��Ũ�� | C�� | ��СC��D��Ũ�� | D�� | ����ϡ������ |
| A�� | ��ˮ�����Ҵ����ױ����屽 | |
| B�� | ��̼������Һ�����Ҵ���������������� | |
| C�� | ��ȼ�շ������Ҵ����������Ȼ�̼ | |
| D�� | �����Ը��������Һ����ױ���������ͻ���ϩ |
| A�� | ��CN��2�ڼ���Һ������CN-��OCN- | |
| B�� | IBr+2NaOH�TNaBr+NaIO+H2O | |
| C�� | �����ڿ�����ȼ�� | |
| D�� | IBr�뱽����ȡ����Ӧ�����屽�͵⻯�� |
| A�� | 1 mol S������Fe��Ӧ��ת�Ƶĵ�����Ϊ3NA�� | |
| B�� | 1.5 mol NO2������H2O��Ӧ��ת�Ƶĵ�����Ϊ2NA�� | |
| C�� | ���³�ѹ�£�46 g��NO2��N2O4������庬�е�ԭ����Ϊ3NA�� | |
| D�� | 100 ml 0.10mol/L�İ�ˮ�У���NH3•H2O ����0.01NA�� |
| A�� | ��a+1.25�� | B�� | ��a+9.6 ��g | C�� | ��a+5.1��g | D�� | ��ȷ�� |
| A�� | �����۵����� | B�� | ԭ�Ӱ뾶 | C�� | Ԫ�صķǽ����� | D�� | ����ϼ� |