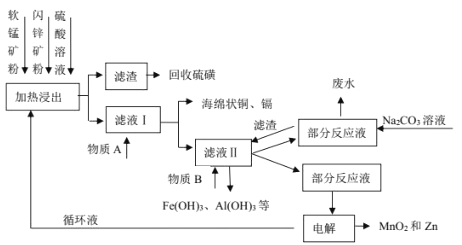
��Ŀ����
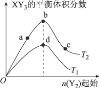
����Ŀ����֪��3H2(g)+N2(g)2NH3(g)��H=-92kJ/mol���ڴ�������ʱ��Ӧ�����е������仯��ͼ��ʾ������������ȷ����
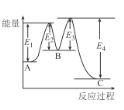
A.��H=E2-E1+E3-E4
B.���������Ӧ����������ɣ����е�һ����Ӧ�����ܷ�Ӧ����
C.�����������H����Ӧ���ʾ������ı�
D.���ܱ������г���3 mol H2��1molN2������������Ӧ���ﵽƽ��ʱ����Ӧ�ų�92 kJ����
���𰸡�B
��������
A. ��A��B�ķ�Ӧ�ʱ�ΪE1-E2����B��C�ķ�Ӧ�ʱ�ΪE3-E4���٣��ڿɵã�A��C����ϸ�˹����A��C�ķ�Ӧ�ʱ�ΪE1-E2+E3-E4��A�����
B. ��ͼ��֪���������Ӧ����������ɣ���һ����Ӧ�Ļ��E1���ڵڶ�����Ӧ�Ļ��E3�����Ե�һ����Ӧ������������ܷ�Ӧ���ʣ�B����ȷ��
C. ���������ͬ�ȳ̶ȵؼӿ����淴Ӧ���ʣ����ʱ���H�ɷ�Ӧ�����������������IJ�ֵ�����ģ�������أ�C�����
D. ��3H2(g)+N2(g)=2NH3(g) ��H=-92kJ/mol��֪��������3 mol H2��1molN2����ų�92 kJ�����������ܱ������г���3 mol H2��1molN2�����ڸ÷�Ӧ�ǿ��淴Ӧ������ȫ��Ӧ�����Է�Ӧ�ų�С��92 kJ������D�����
��ѡB��

����Ŀ���״�(CH3OH)������Ϊ��ɫҺ�壬��Ӧ�ù㷺�Ļ���ԭ�Ϻ�ǰ���ֹ۵�ȼ�ϡ�
(1)��֪��CH4(g)��H2O(g)CO(g)��3H2(g) H����206.0kJ/mol��1
CH4(g)��H2O(g)CH3OH(g)��H2(g) H����77.0kJ/mol��1
��CO��H2��Ӧ����CH3OH(g)���Ȼ�ѧ����ʽ��______________________��
(2)�״������ںϳ�3��5-�����������ӣ���Ӧ���£�
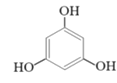 +2CH3OH
+2CH3OH![]()
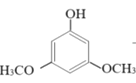 +2H2O
+2H2O
��Ӧ�������ȷ�����״����ټ������ѣ�����õ��л���(�������Ȼ���)����ϴ�ӣ�Ȼ������ᴿ�õ�����״���3��5-�����������ӵIJ����������ʼ�����
���� | �е�/�� | �۵�/�� | �ܽ��� |
�״� | 64.7 | ��97.8 | ������ˮ |
3��5-������������ | 172~175 | 33~36 | �����ڼ״������ѣ�����ˮ |
�ٷ�����״��IJ�����______________________(����ĸ���)��
a������ b����Һ c���ᾧ
��ϴ��ʱ�������ڳ�ȥ�л����е��Ȼ�����Լ���______________________(����ĸ���)��
a��Na2CO3��Һ b��NaHCO3��Һ c��NaOH��Һ
(3)�״�������ʵ�����Ʊ���Ȳ�����(CH![]() C��COOCH3���е�Ϊ103��105��)��
C��COOCH3���е�Ϊ103��105��)��
��ӦΪ��CH��C��COOH+CH3OH![]() CH��C��COOCH3+H2O
CH��C��COOCH3+H2O
ʵ�鲽�����£�
����1���ڷ�Ӧƿ�У�����14g��Ȳ�ᡢ50mL�״���2 mLŨ���ᣬ���裬���Ȼ���һ��ʱ�䡣
����2�����������ļ״�(װ����ͼ��ʾ)��

����3����ӦҺ��ȴ�������ñ���NaCl��Һ��5��Na2CO3��Һ��ˮϴ�ӡ�������л��ࡣ
����4���л��ྭ��ˮNa2SO4������ˡ����ñ�Ȳ�������
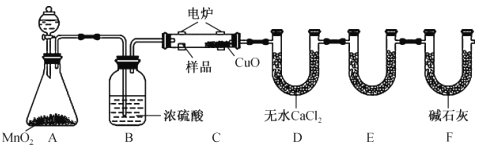
������A��������______________��������ƿ�м������Ƭ��Ŀ����_______________��
�ڲ���3�У���5%Na2CO3��Һϴ�ӣ���Ҫ��ȥ��������______________________��������л���IJ�������Ϊ_____________________��
�۲���4�У�����ʱ������ˮԡ���ȵ�ԭ����______________________��
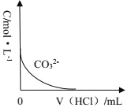
����Ŀ��ijʵ��С���������ữ�ĸ��������Һ�������Һ��Ӧ�ⶨ��λʱ��������CO2������̽��Ӱ�췴Ӧ���ʵ����ء����ʵ�鷽�������
ʵ����� | A��Һ | B��Һ |
�� | 20mL0.1mol��L-1H2C2O4��Һ | 30mL0.02mol��L-1����KMnO4��Һ |
�� | 20mL0.2mol��L-1H2C2O4��Һ | 30mL0.02mol��L-1����KMnO4��Һ |
��1��ͼ1װ����ʢ��A��Һ������������_____��������ͼ1װ�������Եķ���Ϊ_____��

��2�������ữ�ĸ��������Һ�������Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ_____��
��3����ʵ��̽������_____���ضԻ�ѧ��Ӧ���ʵ�Ӱ�죬��ʵ�����40sĩ�ռ���22.4mLCO2(��״����)������40s�ڣ�v(MnO4-)=_____(������Һ���ǰ������ı仯)��
��4��С��ͬѧ��ͼ1�������ռ�װ�ø�Ϊͼ2��ʵ�������ȴ����ʱ����������Һ����ڸ����Һ�棬Ϊ�õ�ȷ���ݣ���ȡ�IJ����ǣ�____��
��5������ͨ���ⶨ��λʱ��������CO2��������ȽϷ�Ӧ���ʣ���ʵ�黹����ͨ���ⶨ____���Ƚϻ�ѧ��Ӧ���ʡ�
��6��ͬѧ����ʵ���з��ַ�Ӧ����������ͼ3��ʾ��̽��t1��t2ʱ�������ʱ�����Ҫԭ������ǣ�____��
A.�÷�Ӧ���� B.���ɵ�Mn2+������� C.K2SO4Ũ������