��Ŀ����
����Ŀ����֬�����̶Ⱦ�������֬�ij�Ĥ�ԡ�������һ�ֳ�Ĥ�����õĴ�������֬�ĺϳ�·�ߣ���ͼ��ʾ��
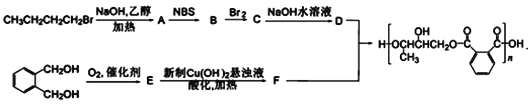
��1��B�ķ���ʽΪC4H7Br����B������˳���칹��B�Ľṹ��ʽΪ___��A��B����ķ�Ӧ������___��
��2��E�к��������ŵ�������___��D��ϵͳ����Ϊ___��
��3������˵����ȷ����___��
A��1mol������C�������3molNaOH B��1mol������E������������Һ��Ӧ����2molAg
C��F������Cu(OH)2����Һ��Ӧ D�����顢1-������������D�зе���ߵ��Ƕ���
��4��д��D��F��һ�����������ɴ�������֬�Ļ�ѧ����ʽ____��
��5��![]() �ķ�������������ͬ���칹����__�֡�
�ķ�������������ͬ���칹����__�֡�
�ٱ��Ķ�ȡ�������� ����FeCl3��Һ����ɫ �ۿɷ�����ȥ��Ӧ
��6����֪������������ܷ������·�Ӧ��
![]()
�Ա������������1��3-���������Ҵ���Ϊԭ�Ϻϳ�![]() ������Ƴ������ķ�Ӧ����___��
������Ƴ������ķ�Ӧ����___��
���𰸡�CH3CHBrCH=CH2 ȡ����Ӧ ȩ�� 1��2��3-������ A n![]() +n
+n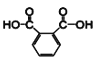
 +(2n-1)H2O 6
+(2n-1)H2O 6 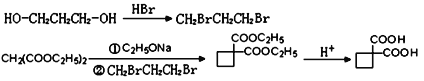
��������
�л��ϳ����⣬����Ҫ������úϳ�·��ͼ���������������Ϣ�����ҳ��м�ֵ����Ϣ���Ӷ��ҳ�����·���еĸ������ʣ�AΪCH3CH2CH=CH2��BΪCH3CHBrCH=CH2��CΪCH3CHBrCHBrCH2Br��DΪ![]() ��EΪ
��EΪ![]() ��FΪ
��FΪ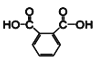 ��
��
��1�������кϳ�·��ͼ��֪��CH3CH2CH2CH2Br�����������Ҵ���Һ�м��ȵ������·�����ȥ��Ӧ����A(CH3CH2CH=CH2)��A(CH3CH2CH=CH2)��NBS����֮������B�� B�ķ���ʽΪC4H7Br������B������˳���칹����BΪCH3CHBrCH=CH2��A��B����ķ�Ӧ������ȡ����Ӧ��
��2��B��Br2�����ӳɷ�Ӧ����C��CΪCH3CHBrCHBrCH2Br��C������������Һ�з���ˮ������D��DΪ()��D������Ϊ1��2��3-��������D��F����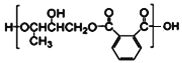 ����֪DΪ
����֪DΪ![]() ��FΪ
��FΪ ������Ϊ
������Ϊ![]() ��O2�ڴ�������������E������EΪ
��O2�ڴ�������������E������EΪ![]() ��E�еĺ���������Ϊȩ����
��E�еĺ���������Ϊȩ����
��3��A��C(CH3CHBrCHBrCH2Br)�����������Ҵ���Һ�з�Ӧ����(CH3CH(OH)CH(OH)CH2OH)������1mol������C�������3molNaOH��A ��ȷ��
B��1mol������E�к���2molȩ��(-CHO)��1mol������E������������Һ��Ӧ����4molAg��B����
C��F�к����Ȼ�(-COOH)����Cu(OH)2����Һ��Ӧ��C����
D�����顢1-������������D(CH3CH(OH)CH(OH)CH2OH)�зе���ߵ��ǻ�����D(CH3CH(OH)CH(OH)CH2OH)��D������
��ѡA��
��4����������֬Ϊ�߷��ӻ��������D��F��һ�������·������۷�Ӧ����Ӧ����ʽΪ��
n![]() +n
+n
 +(2n-1)H2O
+(2n-1)H2O
��5�����������������ٱ��Ķ�ȡ�������˵�������Ϻ�������ȡ����������FeCl3��Һ����ɫ��˵�������к��з��ǻ����ۿɷ�����ȥ��Ӧ
�������������ٱ��Ķ�ȡ������������FeCl3��Һ����ɫ��˵�������ǻ����ۿɷ�����ȥ��Ӧ�����Է�����������![]() ��Ϊͬ���칹�壬�����ϵ�ȡ����Ϊ
��Ϊͬ���칹�壬�����ϵ�ȡ����Ϊ![]() ��
��![]() ��
��![]() ��
��![]() ��ÿһ�������ڡ��䡢�Ը����ֽṹ�����Է���������ͬ���칹����6�֣��ʴ�Ϊ��6��
��ÿһ�������ڡ��䡢�Ը����ֽṹ�����Է���������ͬ���칹����6�֣��ʴ�Ϊ��6��
��6��1,3-������(HOCH2CH2CH2OH)��HBr��Ӧ����BrCH2CH2CH2Br����CH2(COOC2H5)2���Ҵ��ƺ�BrCH2CH2CH2Br��Ӧ����![]() ��
��![]() ������������ˮ��õ�
������������ˮ��õ�![]() ��
��

����Ŀ���������أ�![]() ���ܴ�ʹ����������ȷ������ӷ������������ʵ���Ʊ��������ز��ⶨ�䴿�ȣ�
���ܴ�ʹ����������ȷ������ӷ������������ʵ���Ʊ��������ز��ⶨ�䴿�ȣ�
I.�Ʊ�
����1���Ʊ������ᶡ����![]() ��
��
![]()
��Ӧװ����ͼ1���г�װ����ȥ�������ձ������μ���ϡ���ᡢ����������������Һ������Ӧ��ȫ������ϲ���״���![]() ��
��![]() �Ļ����Һϴ�����Σ���������á�
�Ļ����Һϴ�����Σ���������á�
����2���Ʊ���������
![]()
��Ӧװ����ͼ2���гּ�����װ��·ȥ����������A�м���![]() �Ҵ���Һ��
�Ҵ���Һ��![]() ��������
��������![]() ���������ᶡ��������ԡ���ȣ���Ӧ��ȫ�������ؼ�������������ԡ��ȴ�����ˣ�������ˮ�Ҵ�ϴ�ӣ�������ˮ����ϴ�ӣ��ڿ�������
���������ᶡ��������ԡ���ȣ���Ӧ��ȫ�������ؼ�������������ԡ��ȴ�����ˣ�������ˮ�Ҵ�ϴ�ӣ�������ˮ����ϴ�ӣ��ڿ�������![]() ���
���


��������������£�
���� | ��ɫ��״̬ | �е㣨�棩 | �ܽ��� |
| ��ɫ���� | �����ֽ� | ������ˮ�������Ҵ������������� |
| ��ɫҺ�� | 118 | ����ˮ�����Ҵ������ѻ��� |
| ��ɫ��ɫ��״Һ�� | 78 | ������ˮ�����Ҵ������ѻ��� |
| ��ɫ��״Һ�� | 118 | ��ˮ���Ҵ����ܣ����������� |
��ش�
��1������A������Ϊ_____________.
��2������1�з���������ᶡ���IJ�������Ϊ_____________������1����NaCl��NaHCO3�Ļ����Һϴ�ӵ�Ŀ����__________________________.
��3������2�б�ԡ��ȴ��Ŀ����__________________________������2�и����Ʒ���¶ȿ�����55~60�棬ԭ����__________________________
��4��������߲�Ʒ�Ĵ��ȣ�����_____________�����ţ��н����ؽᾧ��
A.��ˮ�Ҵ� B.��ˮ���� C.ˮ D.�Ҵ���ˮ��Һ
��.�ֹ��ȷ��ⶨ��Ʒ�Ĵ���
ԭ����![]() ��
��![]() ��Ӧ�dz����������ɺ�ɫ������һ�������²�����ɫ��Һ������ȣ����á�
��Ӧ�dz����������ɺ�ɫ������һ�������²�����ɫ��Һ������ȣ����á�![]() ����ȡ�����ȷ����Ʒ��Һ�е�
����ȡ�����ȷ����Ʒ��Һ�е�![]() ���ⶨ�������£�
���ⶨ�������£�
����![]() Ʒ������
Ʒ������![]() ����Һ��
����Һ��
������һ����ͬ�����![]() ����ͬŨ�ȵ�
����ͬŨ�ȵ�![]() ����Һ���ֱ����
����Һ���ֱ����![]() ��������
��������![]() ����Һ��ҡ�ȣ���������ȣ����Ʊ���Һ��
����Һ��ҡ�ȣ���������ȣ����Ʊ���Һ��![]() ������ȵĹ�ϵ���ߣ���ͼ��
������ȵĹ�ϵ���ߣ���ͼ��
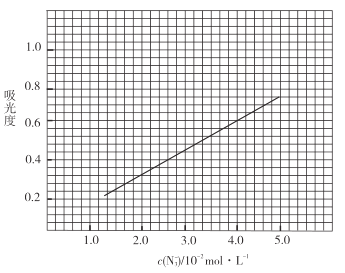
�۲�Ʒ�ⶨ����ȡ0.360g��Ʒ�����![]() ��Һ��ȡ��
��Һ��ȡ��![]() �ڱ����У�����
�ڱ����У�����![]() ��������
��������![]() ����Һ��ҡ�ȣ���������Ϊ0.6��
����Һ��ҡ�ȣ���������Ϊ0.6��
��5��ʵ������![]() ��������
��������![]() ����Һ�ķ���Ϊ_________________.
����Һ�ķ���Ϊ_________________.
��6����Ʒ�Ĵ���Ϊ_________________�������м����![]() ����Һ�����Խ���Ʒ��ȫ��Ӧ�����õIJ�Ʒ����________________���ƫ�ߡ���ƫ�͡�����Ӱ�족����
����Һ�����Խ���Ʒ��ȫ��Ӧ�����õIJ�Ʒ����________________���ƫ�ߡ���ƫ�͡�����Ӱ�족����
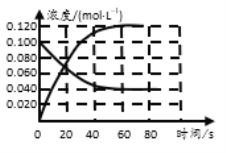
����Ŀ�������£�ijͬѧ�������백ˮ�������ϣ����ǰ������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±����ݱ�����������˵����ȷ���ǣ� ��
ʵ���� | ��ˮŨ��/mol��L��1 | ����Ũ��/mol��L��1 | �����ҺpH |
�� | 0.1 | 0.1 | pH��5 |
�� | c | 0.2 | pH��7 |
�� | 0.2 | 0.1 | pH��7 |
A.����c��0.2
B.�١���������Һ�е�![]() ����>��
����>��
C.����������Һ��c(NH4+)+c(H+)��c(OH��)+c(NH3��H2O��
D.�������û����Һ����ˮ�������c(H��)��1��10��9 mol��L��1