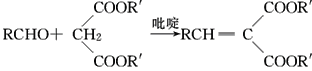
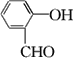
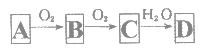��Ŀ����
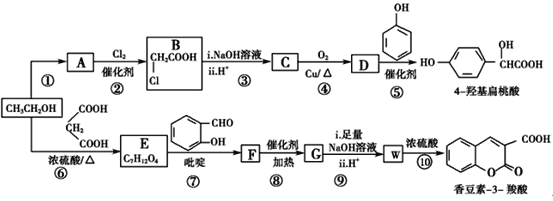
����Ŀ���۴�����ϩ�����ϼ���Ӧ�ù㷺�������Ǹ��л���ĺϳ�·�ߣ�
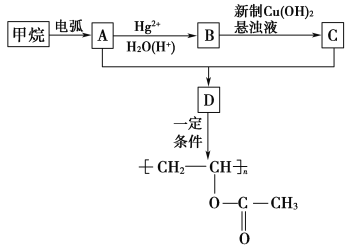
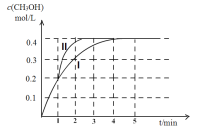
��ʾ���������ڵ绡������������Ȳ����
��CH3C��CH![]() CH3CH2CHO(δ��ƽ)��
CH3CH2CHO(δ��ƽ)��
��ش��������⣺
(1)����ϳ�A�Ļ�ѧ��Ӧ��ԭ��������Ϊ________��
(2)B�Ľṹ��ʽΪ________��
(3)B����C�ķ�Ӧ�г�����Cu(OH)2����Һ���Ҫ��������________��
(4)A��C��Ӧ����D�ķ�Ӧ������________��
(5)д����D����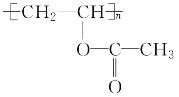 ��Ӧ�Ļ�ѧ����ʽ��________________________________��
��Ӧ�Ļ�ѧ����ʽ��________________________________��
(6)д����ʹ��ɫʯ����Һ����D������ͬ���칹��Ľṹ��ʽ��________________________________��
���𰸡�81.25%CH3CHO���ȼӳɷ�Ӧ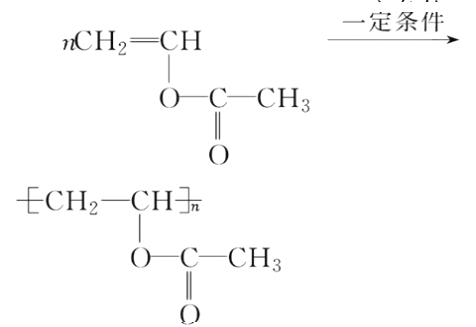 CH2===CHCH2COOH��CH3CH===CHCOOH��
CH2===CHCH2COOH��CH3CH===CHCOOH��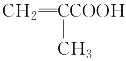
��������
��Ŀ�����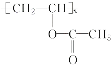 ��֪A��B��C���Ǻ���2��̼ԭ�ӵ��л�������ʾ��Ϣ��֪AΪ��Ȳ��BΪ��ȩ��CΪ���ᡣ�ݴ˷����ɵý��ۡ�
��֪A��B��C���Ǻ���2��̼ԭ�ӵ��л�������ʾ��Ϣ��֪AΪ��Ȳ��BΪ��ȩ��CΪ���ᡣ�ݴ˷����ɵý��ۡ�
(1)����������Ȳ�Ļ�ѧ��Ӧ����ʽΪ2CH4![]() CH��CH��3H2�����Ը÷�Ӧ��ԭ��������Ϊ26��32��100%��81.25%����2��B�Ľṹ��ʽΪ��CH3CHO (3)Bת��ΪCʱ��������������ͭ����Һ��������������ʱ��Ҫ���ȣ��ʴ�Ϊ�����ȣ� (4)��Ȳ�����ᷴӦ����
CH��CH��3H2�����Ը÷�Ӧ��ԭ��������Ϊ26��32��100%��81.25%����2��B�Ľṹ��ʽΪ��CH3CHO (3)Bת��ΪCʱ��������������ͭ����Һ��������������ʱ��Ҫ���ȣ��ʴ�Ϊ�����ȣ� (4)��Ȳ�����ᷴӦ����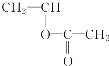 �����Ƿ�����̼̼�����ϵļӳɷ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��(5)D����
�����Ƿ�����̼̼�����ϵļӳɷ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��(5)D����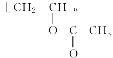 �ķ�ӦΪ�Ӿ۷�Ӧ������ʽΪ��
�ķ�ӦΪ�Ӿ۷�Ӧ������ʽΪ��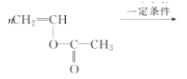
 ��(6)�ܹ�ʹ��ɫʯ����Һ����D��ͬ���칹���к����Ȼ����ʷ���������ͬ���칹�Ľṹ��ʽΪ��CH2=CHCH2COOH��CH3CH=CHCOOH��
��(6)�ܹ�ʹ��ɫʯ����Һ����D��ͬ���칹���к����Ȼ����ʷ���������ͬ���칹�Ľṹ��ʽΪ��CH2=CHCH2COOH��CH3CH=CHCOOH��![]() ��
��

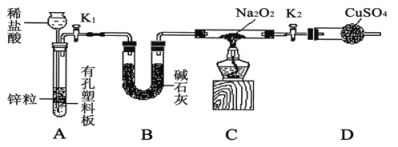
����Ŀ������ֱ���������������Ļ��ᴦ����������ϴ��Һ�к���Fe3+��Ni2+��NO3-��F-��+6�۸��ĺ���������ӵȣ���ͼ���ۺ����ø���ϴ��Һ�Ĺ������̣�
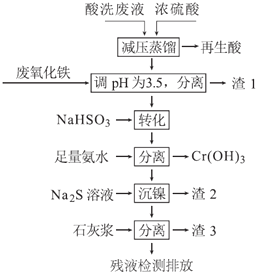
��֪��
�ٽ������ӿ�ʼ�����ͳ�����ȫʱ��pH��
Fe3+ | Ni2+ | Cr3+ | |
��ʼ���� | 1.5 | 6.7 | 4.0 |
������ȫ | 3.4 | 9.5 | 6.9 |
��Ni2+��������ˮ�ķ�ӦΪ��Ni2++6NH3[Ni��NH3��6]2+
��1���������к���______����ȡ��ѹ�����ԭ����______���û�ѧ����ʽ��ʾ����
��2�����÷�����������Ҫ�ɷ�ΪFe2O3�������ռ����pH�ĺô���______��
��3����д����ת����ʱNaHSO3��Cr2O72-������Ӧ�����ӷ�Ӧ����ʽ��______��
��4����֪[Ni��NH3��6]2+Ϊ�ѵ����������ӣ������������ӷ���ʽΪ��______��
��5������3����Ҫ�ɷ�ΪCa��OH��2��_________________________��
��6������⣬���IJ�Һ��c��Ca2+��=0.004molL-1�����Һ��F-Ũ��Ϊ______mgL-1��[��֪Ksp��CaF2��=4��10-11mol3L-3.