��Ŀ����
16��д�����л�ѧ��Ӧ����ʽ����ע����Ӧ���ͣ���1���Ҵ���صķ�Ӧ��2CH3CH2OH+2K��2CH3CH2OK+H2����������
��2����ϩ����ˮ��ӦCH2=CH2+Br2��CH2BrCH2Br
��3������������ӦC6H6+HNO3$��_{��}^{Ũ����}$C6H5NO2+H2O
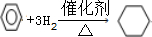
��4������Ni�����������ȵ��������������ķ�Ӧ
��5�����������NaOH���������·�����ӦCH3COOCH3+NaOH$\stackrel{H_{2}O}{��}$CH3COONa+CH3OH��
���� ��1���Ҵ���صķ�Ӧ�����Ҵ��ƺ�������
��2����ϩ����ˮ�����ӳɷ�Ӧ���������飻
��3������������Ũ������������¼���������������
��4������Ni�����������ȵ������������������ӳɷ�Ӧ���ɻ����飻
��5�����������NaOH���������·���ˮ�ⷴӦ���������ƺͼ״���
��� �⣺��1���Ҵ���صķ�Ӧ�����Ҵ��ƺ�����������ʽ��2CH3CH2OH+2K��2CH3CH2OK+H2���������û���Ӧ��
�ʴ�Ϊ��2CH3CH2OH+2K��2CH3CH2OK+H2�����û���Ӧ��
��2����ϩ����ˮ�����ӳɷ�Ӧ���������飬����ʽ��CH2=CH2+Br2��CH2BrCH2Br�����ڼӳɷ�Ӧ��
�ʴ�Ϊ��CH2=CH2+Br2��CH2BrCH2Br���ӳɷ�Ӧ��
��3���������ᷢ��ȡ����Ӧ����������������ʽ��C6H6+HNO3$��_{��}^{Ũ����}$C6H5NO2+H2O������ȡ����Ӧ��
�ʴ�Ϊ��C6H6+HNO3$��_{��}^{Ũ����}$C6H5NO2+H2O��ȡ����Ӧ��
��4������Ni�����������ȵ��������������ķ�Ӧ���ɻ����飬����ʽ�� �����ڼӳɷ�Ӧ��
�����ڼӳɷ�Ӧ��
�ʴ�Ϊ�� ���ӳɷ�Ӧ��
���ӳɷ�Ӧ��
��5�����������NaOH���������·���ˮ�ⷴӦ���������ƺͼ״�������ʽ��CH3COOCH3+NaOH$\stackrel{H_{2}O}{��}$CH3COONa+CH3OH������ȡ����Ӧ��
�ʴ�Ϊ��CH3COOCH3+NaOH$\stackrel{H_{2}O}{��}$CH3COONa+CH3OH��ȡ����Ӧ��
���� ���⿼�����л���ѧ��Ӧ����ʽ����д����ȷ�л���Ľṹ�������ǽ���ؼ���ע�ⷴӦ��������Ӧ���ͣ�

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�| A�� | ��ʪ��ĺ�ɫʯ����ֽ���鰱�� | |
| B�� | ������ͭ��Ũ���ᷴӦ��һ���������� | |
| C�� | �������Ż�ʱ�����ö�����̼�������� | |
| D�� | Na2CO3�����ȶ��Դ���NaHCO3 |
| A�� | Al��Al2O3��Al��OH��3��NaAlO2 | B�� | Si��SiO2��H2SiO3��Na2SiO3 | ||
| C�� | N2��NO��NO2��HNO3 | D�� | S��SO3��H2SO4��MgSO4 |
| A�� | CH2=CH2+Br2��BrCH2CH2Br | |
| B�� | 2CH3CH2OH+O2$��_{��}^{����}$2CH3CHO+2H2O | |
| C�� | 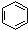 +HNO3$\stackrel{Ũ�����}{��}$ +HNO3$\stackrel{Ũ�����}{��}$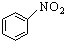 +H2O +H2O | |
| D�� | C6H12O6$��_{��}^{����}$2C2H5OH+2CO2�� |
| A�� | DԪ�ش���Ԫ�����ڱ��е�3���ڵڢ��� | |
| B�� | ����Ԫ�ص�ԭ�Ӱ뾶��A��B��C��D | |
| C�� | B��D������������У�B��D����ԭ��֮���Ϊ�������� | |
| D�� | һ�������£�B�������û���D���ʣ�C�������û���A���� |
| A�� | �����·�Ӧ4Fe��OH��2��s��+2H2O��l��+O2��g���T4Fe��OH��3��s���ġ�H��0����S��0 | |
| B�� | 500�桢30 MPa�£���0.5 mol N2��1.5 mol H2�����ܱ������г�ַ�Ӧ������19.3 kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��$?_{500�桢30MPa}^{����}$2NH3��g������H=-38.6 kJ•mol-1 | |
| C�� | FeCl3��Һ��ͨ��SO2����Һ��ɫ��ȥ��2Fe3++SO2+2H2O�T2Fe2++SO42-+4H+ | |
| D�� | ������Һ��ͨ��CO2����Һ����ǣ�2C6H5O-+CO2+H2O-��2C6H5OH��+CO32- |
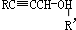
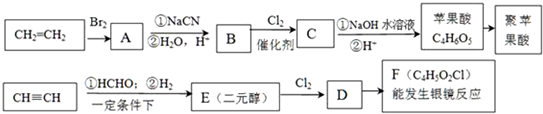
 ��
��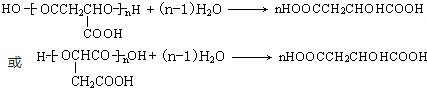 ��
��